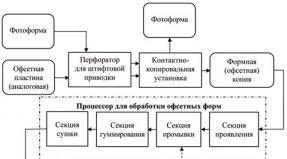
Equipment for nitrogen production. Obtaining nitrogen Producing nitrogen from air
Since free nitrogen is contained in the atmosphere, its production comes down to separation from oxygen and other components of the air. This is done by gradual evaporation of liquid air in special installations, and at the same time oxygen and inert gases are also produced.
Nitrogen is a colorless and odorless gas (mp -210°C, bp -196°C). Its solubility in water is low - about 2% by volume. The nitrogen molecule is diatomic and does not noticeably disintegrate into atoms even at very high temperatures.
Free nitrogen is chemically very inert. Under normal conditions, it does not react with metalloids or metals (except Li). With increasing temperature, its activity increases mainly in relation to metals, with some of which it combines when heated, forming nitrides of these metals (for example, Mg 3 N 2).
3Mg + N 2 = Mg 3 N 2
The use of free nitrogen, as such, is quite limited. It is mainly used to fill electric lamps. Nitrogen compounds are of great importance for biology and are used in a variety of industries. The largest quantities of them are consumed as mineral fertilizers and in the production of explosives.
The main starting product for the industrial production of nitrogen compounds is free nitrogen from the air. Its transfer to the bound state is carried out mainly by the method of ammonia synthesis, developed in 1913.
Application to a reversible reaction
N 2 + ZN 2< = >2NH 3 + 22 kcal
The principle of shifting equilibrium shows that the most favorable conditions for the formation of ammonia are the lowest possible temperature and the highest possible pressure. However, even at 700°C the reaction rate is so low (and therefore equilibrium is established so slowly) that there can be no question of its practical use. On the contrary, at higher temperatures, when the equilibrium state is quickly established, the ammonia content in the system becomes negligible. Thus, the technical implementation of the process under consideration turns out to be impossible, since by accelerating the achievement of equilibrium with the help of heating, we simultaneously shift the equilibrium position to an unfavorable side.
There is, however, a means to accelerate the achievement of an equilibrium state without simultaneously shifting the equilibrium. This often helps with the use of a suitable catalyst.
Metallic iron (with an admixture of Al 2 O 3 and K 2 O) turned out to work well in this case.
The process of ammonia synthesis is carried out at temperatures of 400-550°C (on a catalyst) and pressures of 100-1000 at.
In this case, equilibrium is established quite quickly. After ammonia is separated from the gas mixture, the latter is reintroduced into the cycle. Over a quarter of a century, from 1913 to 1938, the annual world production of nitrogen bound in this way increased from 7 tons to 1,700 thousand tons. Currently, ammonia synthesis is the main industrial method for producing bound nitrogen.
Of significantly less industrial importance is the cyanamide method developed in 1901, which is based on the fact that at high temperatures calcium carbide (obtained by heating a mixture of lime and coal in an electric furnace) reacts with free nitrogen according to the equation
CaC 2 + N 2 = CaCN 2 + C + 70 kcal
Calcium cyanamide (Ca = N-C?N) obtained in this way is a gray (from carbon impurity) powder. When exposed to superheated (i.e. heated above 100°C) water vapor, it decomposes, releasing ammonia:
CaCN 2 + 3H 2 O = CaCO 3 + 2NH 3
The furnace for producing calcium cyanamide is a cylinder made of refractory material, along the axis of which runs a pipe with a heating winding inside. After loading the furnace with crushed CaC 2, it is tightly closed and nitrogen is supplied to it. Since the formation of cyanamide is accompanied by the release of heat, it is enough to heat the initial mixture to 800°C, and then the reaction proceeds on its own. During the period from 1913 to 1938, the annual world production of fixed nitrogen using the cyanamide method increased from 38 thousand tons to 300 thousand tons.
The NH 3 molecule has the shape of a triangular pyramid. Since the electrons of the H-N bonds are quite strongly shifted from hydrogen to nitrogen (pNH = 0.28), the ammonia molecule as a whole is characterized by significant polarity (dipole length 0.31 A).
Ammonia is a colorless gas (mp -78°C, bp -33°C) with a characteristic pungent odor of “ammonia”. Its solubility in water is greater than that of all other gases: one volume of water absorbs about 1200 volumes of NH 3 at 0°C, and about 700 at 20°C. The commercial concentrated solution typically has a density of 0.91 and contains 25% NH 3 by weight.
Like water, liquid ammonia associates primarily through the formation of hydrogen bonds. It is a good solvent for many inorganic and organic compounds.
The association of liquid ammonia is associated with its high heat of vaporization (5.6 kcal/mol). Since the critical temperature of NH 3 is high (+ 133 ° C) and when it evaporates a lot of heat is removed from the environment, liquid ammonia can serve as a good working substance for refrigeration machines. When the piston moves to the right, NH 3 heated by compression enters the coil, cooled externally with water (or air). Cooled ammonia, already at the existing pressure in the system (7-8 atm), is compressed and flows into the receiver, from which liquid ammonia enters the coil, where it evaporates due to the vacuum in this part of the system. The heat required for evaporation is absorbed from the space surrounding the coil. Consecutive repetition of the entire cycle of processes creates continuous cooling of the space surrounding the coil.
For the chemical characteristics of ammonia, reactions of three types of addition, hydrogen substitution and oxidation are of primary importance.
The most typical reactions for ammonia are addition reactions. In particular, when it acts on many salts, crystalline ammonia compounds of the composition CaCl 2 · 8NH 3, CuSO 4 · 4NH 3, etc. are formed, similar in the nature of formation and stability to crystalline hydrates.
When ammonia is dissolved in water, ammonium hydroxide is partially formed:
NH 3 + H 2 O< = >NH4OH
In this compound, the ammonium radical (NH 4) plays the role of a monovalent metal. Therefore, the electrolytic dissociation of NH 4 OH proceeds according to the main type:
NH4OH< = >NH 4 + + OH -
Combining both equations, we obtain a general idea of the equilibria that take place in an aqueous ammonia solution:
NH 3 + H 2 O< = >NH4OH< = >NH 4 + + OH -
Because of the presence of these equilibria, an aqueous solution of ammonia (often called simply "ammonia") smells strongly of it. Due to the fact that this solution contains relatively few OH ions, NH 4 OH is considered a weak base.
The addition of acids leads to a shift of the above equilibria to the right (due to the binding of OH ions) and to the formation of ammonium salts, for example, according to the equation:
NH 4 OH + HCl = H 2 O + NH 4 Cl
These salts are also formed by the direct interaction of ammonia with acids, for example, by the reaction:
NH3 + HCl = NH4Cl
Both the ammonium ion itself (NH 4 +) and most of its salts are colorless. Almost all of them are highly soluble in water and strongly dissociated in solutions.
When ammonium salts are heated, they decompose quite easily. The nature of decomposition is determined by the properties of the anion-forming acid. If the latter is an oxidizing agent, ammonia is oxidized according to the reaction, for example:
NH 4 NO 2 = 2H 2 O + N 2
If the acid is not an oxidizing agent, the nature of decomposition is determined by its volatility at the decomposition temperature. From salts of non-volatile acids (for example, H 3 PO 4), only ammonia is released, but if the acid is volatile (for example, HCl), then upon cooling it combines again with NH 3. The result of such decomposition and subsequent recombination practically boils down to the fact that the salt in question (for example, NH 4 Cl) sublimes.
Under the influence of ammonium salts: silt alkalis, ammonia is released according to the reaction, for example:
NH 4 Cl + NaOH = NaCl + NH 4 OH = NaCl + NH 3 + H 2 O
This can be used for the laboratory production of ammonia, as well as for the discovery of NH ions in solution: alkalis are added to the latter and then the released ammonia is detected by smell or its effect on wet litmus paper.
Ammonium derivatives are of great practical importance. Its hydroxide (NH 4 OH) is one of the most important chemical reagents, diluted solutions of which (“ammonia”) are sometimes also used in the household (when washing clothes and removing stains). Ammonium chloride (“ammonia”) reacts with metal oxides at high temperatures, exposing a clean metal surface. This is the basis for its use in metal soldering. In electrical engineering, NH 4 Cl is used for the manufacture of “dry” galvanic cells. Ammonium nitrate (NH 4 NO 3) is the basis of complex nitrogen fertilizers and is also used for the preparation of some explosive mixtures. Ammonium sulfate [(NH 4) 2 SO 4 ] is consumed in large quantities by agriculture as a nitrogen fertilizer. Ammonium carbonate (NH 4 HCO 3) is used in baking (mainly in confectionery production). Its use is based on the fact that when heated it easily decomposes according to the following scheme:
NH 4 HCO 3 = NH 3 ^ + H 2 O + CO 2 ^
and the resulting gases give the dough the necessary porosity. Ammonium sulphide [(NH 4) SO 4 ] is one of the main reagents in analytical chemistry. Ammonium compounds play an important role in some production processes of the chemical industry and are widely used in laboratory practice.
Commercial ammonia usually contains about 10% ammonia. It also has medical uses. In particular, inhaling its vapors or taking it orally (3-10 drops per glass of water) is used to relieve severe intoxication. Lubricating the skin with ammonia weakens the effect of insect bites. When removing stains, the following compositions (by volume) give good results in many cases:
- a) 4 parts of ammonia, 5 parts of ether and 7 parts of wine alcohol;
- b) 10 parts of ammonia, 7 parts of wine alcohol, 3 parts of chloroform and 80 parts of gasoline.
The explosive decomposition of ammonium nitrate proceeds mainly according to the equation:
2NH 4 NO 3 = 4H 2 O + O 2 + 57 kcal
Ammonal, sometimes used in blasting practice, is a close mixture of NH 4 NO 3 (72%), aluminum powder (25%) and coal (3%). This mixture explodes only from detonation.
Hydrogen substitution reactions are less typical for ammonia than the addition reactions discussed above. However, at high temperatures it is capable of replacing its hydrogens with metal, for example, by the reaction:
2Al+2NH 3 = 2AlN + ZN 2
It is by heating metals in an ammonia atmosphere that nitrides are most often obtained. The latter are solid substances, mostly very resistant to heat. Active metal nitrides decompose more or less easily with water, releasing ammonia, for example, according to the following scheme:
Mg 3 N 2 + 6H 2 O = 3Mg(OH) 2 + 2NH 3 ^
Nitrides of low-active metals with respect to water are, as a rule, very stable.
Due to the non-volatility of nitrides and their insolubility in any of the known solvents, methods for determining molecular weights applicable to them do not yet exist. Therefore, only the simplest formulas of nitrides are known. In many of them the apparent valency of the metal is compatible with its usual values. In other cases, the simplest formula itself indicates the complexity of the molecular structure. The first type includes, for example, Mn 3 N 2, the second - Cr 2 N.
When only two hydrogen atoms are replaced in an ammonia molecule, imides are obtained, and when only one is replaced, metal amides are obtained. The former contain a divalent radical = NH (imino group), the latter contain a monovalent radical - NH 2 (amino group). For example, when passing dry NH 3 over heated sodium metal according to the reaction
2Na + 2NH 3 = 2NaNH 2 + H 2
colorless sodium amide is formed, which is a typical salt with the NH 2 anion. It decomposes with water according to the equation:
NaNH 2 + H 2 O = NH 3 + NaOH
Sodium amide is used in organic syntheses.
Along with metal derivatives, products of substitution of ammonia hydrogens by halogen are known. An example is nitrogen chloride (NCl 3), which is formed in the form of yellow oily drops when chlorine acts on a strong solution of ammonium chloride:
NH 4 Cl + 3Cl 2 = 4HCl + NCl 3
NCl 3 vapors (mp. -27°C, bp. 71°C) have a pungent odor. Already when heated above 90°C (or impact), nitrogen chloride breaks down into elements with a strong explosion.
When iodine acts on a strong solution of NH 3, a dark brown precipitate of so-called nitrogen iodide is released, which is a mixture of NJ 3 with NHJ 2 and NH 2 J. Nitrogen iodide is extremely unstable and, in its dry form, explodes at the slightest touch.
The product of replacing one of the hydrogens of ammonia with a hydroxyl group is hydroxylamine (NH 2 OH). It is formed during the electrolysis of nitric acid (with a mercury or lead cathode) as a result of the reduction of HNO 3 according to the scheme:
HNO 3 + 6H => 2H 2 O + NH 2 OH
Hydroxylamine is colorless crystals. It is used mainly as a reducing agent.
With acids, hydroxylamine (mp 33°C) gives salts, of which chloride (NH 2 OH·HCl) is its usual commercial preparation. All hydroxylamine compounds are poisonous and are generally highly soluble in water. Oxidizing agents convert hydroxylamine either to N2 or N2O, for example, by the reactions:
- 2NH 2 OH + HOCl = N 2 +HCl + 3H 2 O
- 6NH 2 OH + 4HNO 3 = 3N 2 O + 4NO + 11H 2 O.
Like hydrogen substitution, oxidation reactions for ammonia are relatively uncommon. It does not burn in air, but when ignited in an oxygen atmosphere it burns according to the equation:
4NH 3 + ZO 2 = 6H 2 O + 2N 2
Chlorine and bromine react vigorously with ammonia according to the following scheme:
2NH 3 + ZG 2 = 6NG + N 2
They also oxidize ammonia in solution. In relation to most other oxidizing agents, NH 3 is stable under normal conditions. The most important product of the partial oxidation of ammonia is hydrazine (N 2 H 4), formed by the reaction:
2NH 3 + NaOCl = H 2 O + N 2 H 4 + NaCl
As can be seen from the equation, under the action of an oxidizing agent, each ammonia molecule loses in this case one hydrogen atom, and the remaining NH 2 radicals combine with each other. The structural formula of hydrazine will therefore be H 2 N-NH 2.
Hydrazine is a colorless liquid that is miscible with water in any proportion. It finds use as a reducing agent.
By adding acids, hydrazine (mp 2°C, bp 114°C) forms two series of salts, for example N 2 H 4 HCl and N 2 H 4 2 HCl. It is usually oxidized to free nitrogen (for example, by the reaction:
2K 2 Cr 2 O 7 + 3N 2 H 4 + 8H 2 SO 4 = 2K 2 SO 4 + 2Cr 2 (SO 4) 3 + 3N 2 + 14H 2 O)
Hydrazine vapor mixed with air can burn according to the reaction
N 2 H 4 + O 2 => 2H 2 O + N 2 + 149 kcal
This is the basis for its use as rocket fuel. Hydrazine and all its derivatives are poisonous.
When hydrazine reacts with nitrous acid according to the scheme
N 2 H 4 + HNO 2 = 2H 2 O + HN 3
Hydronitric acid (H-N = N?N) is formed, which is a colorless volatile liquid with a pungent odor. The strength of hydronitric acid is close to acetic acid, and the solubility of salts (azides) is similar to hydrochloric acid. Like HN 3 itself, some azides explode violently when heated or shocked. This is the basis for the use of lead azide as a detonator, i.e. a substance whose explosion causes instantaneous decomposition of other explosives.
The acid function of HN 3 (mp. -80°C, bp. +36°C) is characterized by the value K = 3 ·10-5. Its explosive disintegration follows the reaction:
2NH 3 = H 2 + 3N 2 + 142 kcal
Anhydrous hydronitric acid can explode even by simply shaking the vessel. On the contrary, in a dilute aqueous solution it practically does not decompose during storage. HN 3 vapors are very toxic, and its aqueous solutions cause inflammation of the skin. Azides are usually colorless.
In laboratories, nitrogen can be obtained by the decomposition reaction of ammonium nitrite:
NH 4 NO 2 > N 2 ^ + 2H 2 O+Q
The reaction is exothermic, releasing 80 kcal (335 kJ), so the vessel must be cooled while it occurs (although ammonium nitrite must be heated to start the reaction).
In practice, this reaction is performed by adding dropwise a saturated solution of sodium nitrite to a heated saturated solution of ammonium sulfate, and the ammonium nitrite formed as a result of the exchange reaction instantly decomposes.
The gas released in this case is contaminated with ammonia, nitrogen oxide (I) and oxygen, from which it is purified by successively passing through solutions of sulfuric acid, iron (II) sulfate and over hot copper. The nitrogen is then dried.
Another laboratory method for producing nitrogen is heating a mixture of potassium dichromate and ammonium sulfate (in a ratio of 2:1 by weight). The reaction proceeds according to the equations:
K 2 Cr 2 O 7 + (NH 4) 2 SO 4 = (NH 4) 2 Cr 2 O 7 + K 2 SO 4
(NH 4) 2 Cr 2 O 7 >(t) Cr 2 O 3 + N 2 ^ + 4H 2 O
The purest nitrogen can be obtained by decomposition of metal azides:
2NaN 3 >(t) 2Na + 3N 2 ^
The so-called “air” or “atmospheric” nitrogen, that is, a mixture of nitrogen with noble gases, is obtained by reacting air with hot coke:
O 2 + 4N 2 + 2C > 2CO + 4N 2
This produces the so-called “generator” or “air” gas - raw material for chemical synthesis and fuel. If necessary, nitrogen can be separated from it by absorbing carbon monoxide.
Molecular nitrogen is produced industrially by fractional distillation of liquid air. This method can also be used to obtain “atmospheric nitrogen”. Nitrogen plants that use adsorption and membrane gas separation methods are also widely used.
One of the laboratory methods is passing ammonia over copper (II) oxide at a temperature of ~700°C:
2NH3 + 3CuO > N2^ + 3H2O + 3Cu
Ammonia is taken from its saturated solution by heating. The amount of CuO is 2 times greater than calculated. Immediately before use, nitrogen is purified from oxygen and ammonia by passing over copper and its oxide (II) (also ~700°C), then dried with concentrated sulfuric acid and dry alkali. The process is quite slow, but it is worth it: the gas obtained is very clean.
A nitrogen production plant is a set of equipment that concentrates nitrogen from atmospheric air. The maximum nitrogen concentration at the outlet is 99.9999%. This indicator can be adjusted depending on the purpose of the gas.
Adsorption generator
Production occurs through the supply of compressed air under pressure, which is pumped by a screw air compressor. The generator is equipped with a filtration system and a dryer. The air dryer can be either refrigerated or adsorption, depending on the purpose and the required nitrogen concentration. During the production process, compressed air passes through coarse and fine cleaning and drying, while a dew point of +3C is reached and the air class complies with ISO 8573-1:2010-1.4.1. Then the air is supplied after multi-stage filtration to the generator. At the outlet of the nitrogen equipment we obtain finished gas under pressure up to 10 bar. The station consists of two columns containing an adsorbent to absorb the appropriate type of gas. Once every 8-15 years, the adsorbent needs to be replaced, depending on operating conditions.
Advantages of adsorption type nitrogen generators:
- large work resource;
- quick start/stop;
- ease of operation;
- compactness;
- high reliability;
- no operator control required during operation;
- full automation;
- possibility of remote control via the General Gas company website.
Membrane generator
The separation of gases occurs due to a gas separation membrane. Filtered air passes through the membrane module. The flow passes through thousands of selective fibers. Nitrogen comes out from the back of the membrane, and oxygen comes out through its walls.
Nitrogen production requires the qualified installation of the entire complex of equipment, which requires compliance with safety standards.
Equipment for the production of nitrogen, which is manufactured by General Gas, includes components from certified manufacturers and is tested to ensure compliance with high standards of quality and safety of industrial units.
This equipment allows you to achieve high energy efficiency in the production of nitrogen, which is used in various industries:
- electronic;
- food;
- metalworking;
- pharmaceutical;
- metallurgical;
- oil and gas;
- petrochemical and chemical.
By purchasing equipment for nitrogen production from our company, you receive favorable prices, a guarantee, fast delivery and installation.
How to choose equipment for nitrogen production?
In order to choose the type of air separation unit, you need to understand what they are:
To obtain technical gases from atmospheric air, there are currently three types of air separation units (ASU):
- Air separation units of cryogenic type.
- Air separation units of adsorption type.
- Membrane air separation units.
A cryogenic type ASU is a complex of equipment that carries out sequential processing and cooling of atmospheric air to cryogenic temperatures and subsequent separation by rectification into its components: oxygen, nitrogen, argon, krypton, xenon.
Cryogenic ASUs are divided into:
- Small = 30 ÷ 300 m³/hour;
- Average = 300 ÷ 3000 m³/hour;
- High > 3000 m³/hour;
An adsorption type ASU is a set of equipment that separates atmospheric air by passing it through a molecular sieve, which by its structure can retain gas molecules. Adsorption ASUs are designed to obtain the main separation products in a gaseous state:
- Oxygen;
- Nitrogen.
The productivity of adsorption units is not limited and depends on the number of modules used, but there are restrictions on the concentration (purity) of separation products:
- Output oxygen gas concentration up to 98%
- Output nitrogen gas concentration up to 99.9995%
Membrane ASUs are a set of equipment that separates compressed air by passing it through membrane modules, in which it is separated into its main components: nitrogen and oxygen. Membrane ASUs are designed to obtain separation products in a gaseous state. The performance of membrane installations depends on the number of membrane modules used.
- The concentration of oxygen gas at the outlet is up to 90%.
- The concentration of nitrogen gas at the outlet is up to 99.5%.
Also, to obtain gaseous gases at the point of consumption, cryogenic gasifiers are used, which in turn convert the liquid cryo-product (nitrogen, oxygen or argon) into a gaseous state.
In which case, which ASU should be used to obtain NITROGEN?
In order to select an ASU, you need to know the following parameters:
- Nitrogen gas consumption m³/hour;
- Nitrogen pressure, bar;
- Nitrogen concentration, % or residual oxygen fraction;
- Peak consumption, number of “peaks”, duration and frequency;
- Installation placement option (outdoors, indoors...);
- Existing communications;
- Distance from the object;
- Work schedule (consumption);
- Availability of staff.
Let's look at a visual graph:
On the chart:
- Delivery in cylinders
- Delivery liquid or in cylinders
- Delivery in liquid
- Cryogenic ASUs
Choosing a nitrogen source is a complex and demanding task; the efficiency of production processes and the cost of the final product depend on the correct choice.
At the moment, the market for adsorption nitrogen generators is rapidly developing and in areas where gaseous nitrogen is required, this type of generators shows the lowest cost of production nitrogen, which is ~0.3 kW per 1 cubic meter of nitrogen.
Technology used
The generator extracts nitrogen from ambient air and other gases using pressure swing adsorption technology. During the pressure swing adsorption process, compressed clean ambient air is introduced to a molecular sieve, which allows nitrogen to pass in as a product gas but adsorbs other gases. The screen allows the adsorbed gases to escape to the atmosphere when the outlet valve is closed and the filtration pressure returns to ambient pressure. The filter layer is then purged with nitrogen before fresh compressed air is introduced for a new production cycle. In order to guarantee a constant product flow, nitrogen generators use two molecular filter layers that are connected alternatively between the adsorption and regenerating phases. Under normal operating conditions and with proper maintenance, molecular filter layers have an almost indefinite service life. Pressure swing adsorption technology has several international patents and meets market standards in performance and efficiency.
Equipment layout
In order for the nitrogen generator to work automatically, the following components are required:
Compressed air supply
Supply of a certain amount of compressed air and a certain quality, described in the offer section. The minimum amount of free compressed air supply in m 3 /min at 20°C is equal to the average air consumption of the nitrogen generator in Nm 3 /min, increased by an appropriate percentage to compensate for the influence of ambient air and the design tolerances of the air compressor under design conditions. The air compression system will be included in the scope of supply, which will consist of an air compressor and a refrigerated air dryer.
Air filters
A set of coarse and high purity filters and an activated carbon filter are always included in the scope of delivery. Air filters must be installed between the compressed air supply and the air receiver to ensure that the nitrogen generator will receive the required minimum amount of nitrogen.
Air receiver
The air receiver is installed between the air filters and the nitrogen generator. The main task of the air receiver is to guarantee the supply of a sufficient amount of fresh air to the newly restored filter layer of the nitrogen generator in a short period of time. If the compressed air system is included in the scope of supply, the dimensions of the air receiver volume will vary to those satisfactory for the process and air compression (max. load / no-load cycles).
Nitrogen receiver
The nitrogen generator's product flow is collected in one nitrogen receiver. The nitrogen receiver must be installed in close proximity to the nitrogen generator. The presence of a nitrogen receiver guarantees sufficient back pressure for the process and a constant flow of nitrogen to the end customer. Unless specifically stated, the volume of the nitrogen receiver is calculated based on the assumption of constant consumption dynamics of the Customer's application over a long period of time.
Advantages:
Safety
Low operating pressures, safe storage. There is no need for heavy high pressure gas cylinders. The hazardous storage of liquid nitrogen can be eliminated.
Economy
There are no distribution or processing costs. Producing nitrogen on site (industrial site) with nitrogen generators saves the cost of processing and storage in high-pressure gas cylinders and prevents rental costs, transportation and evaporation losses for users.
Low operating costs.
The proposed process has more efficient separation than other systems on the market. This reduces the need for air supply, resulting in 10 - 25% energy savings compared to comparable systems. By keeping rotating parts to a minimum and using high quality components, maintenance costs remain low throughout the life of the generator.
Convenience
Easy to install and maintain. Nitrogen generators have air inlet and nitrogen outlet on the same side. This means easy installation, even at small workshop angles. High reliability due to reduced number of rotating parts and high quality components.
Guaranteed nitrogen quality
No risk of insufficient nitrogen purity, automatic resumption of the process. Nitrogen generators have a unique control system: if the purity of nitrogen does not match the specified value, the PLC automatically closes the nitrogen production flow to the outlet of the customer's application and opens the off-spec nitrogen release valve. The system will try to start the process, and when the nitrogen purity reaches the desired result, the relief valve will close and the nitrogen intake valve will open again. Fully automatic and unattended procedure, no manual restart required.
Design conditions
| Performance | 1000 Nm³/h (2 x 500 Nm³/h) |
| Residual oxygen content and gas produced | £0.1% vol. |
| Product supply pressure | 5.5 barg |
| Product dew point | £-40 °C at 1 atm. |
| Inlet air flow | 4392.0 Nm³/h (2 x 2196.0 Nm³/h) |
| Max. noise level | 85 dB(A) at 1 meter |
| Planned environmental conditions | |
| Barometric pressure | 1013.25 mbar a |
| Location height | 0 m above sea level |
| Air temperature | 20 °C |
| Relative humidity | 65% |
| Inlet air consumption | |
| Pressure | |
| Temperature | |
| Group composition of hydrocarbons | <6,25 мг/м³ или 5 ppmV |
| Particles | <5 мг/м³ при макс. 3 мкм |
| Dew point | £+3 °C at 7 barg. |
| Site conditions | |
| Power supply system | 400 / 230 V AC, 50 Hz |
| Zone classification | unclassified area/safe area |
| accommodation | in a room with good ventilation |
Data given for ideal operating conditions, tolerance ±5%

Dimensions, weight
Energy settings
Tolerance on all specified values: ± 10%
Scope of delivery
4 air compressors
- oil injection rotary screw compressor
4 air dryers
- refrigerated air dryer
2 air receivers
- carbon steel vertical pressure vessel
- volume: 3000 l
compressed air filters
Two sets of external compressed air filters are installed in front of the air receiver, the set consists of the following filters:
- one coalescing primary filter (efficiency 99.9999%, 1.0 µ - ≤ 0.5 mg/m³) with a float-type condensate drain device;
- one coalescing fine filter (efficiency 99.9999%, 0.01 µ - ≤ 0.1 mg/m³) with a float-type condensate drain device;
- one activated carbon filter (residual oil ≤ 0.005 mg/m³).
two nitrogen generators
Two nitrogen generators, fully pre-wired, mounted on a painted carbon steel frame, each equipped with the following components:
- 6 adsorption towers, each filled with carbon molecular sieve. The carbon molecular sieve will be made in the USA, Europe or Japan. Sieves made in China or India are not used;
- Exhaust gas silencer, installed to muffle the exhaust gas to the design noise level;
- Set of electro-pneumatic process valves and throttles, incl. solenoid valves;
- 1 substandard nitrogen purge line with solenoid controlled control valve;
- A set of safety valves adjusted to the appropriate pressure level;
- All pipelines and electrical cables for connection;
- Local pressure sensors;
- One (1) control system for fully automatic generator operation, fully internally wired, consisting of the following:
- One PLC (Rockwell/Allen Bradley Micro 850 PLC) with Ethernet/IP connection for communication with the customer's remote control system;
- One touchscreen graphical user interface (Rockwell/Allen Bradley C400) displaying real-time values of relevant parameters and possible alarm messages for direct diagnostics;
- All piping, valves, instrumentation and turnkey control system mounted on a carbon steel frame;
- One (1) stand-alone residual nitrogen analyzer with zirconia sensor;
- One self-contained electronic product flow meter.
two (2) nitrogen receivers
- vertical high pressure vessel made of carbon steel;
- safety valves set to the appropriate pressure level
- volume: 3000 l
- max operating pressure: 11.0 barg
Applicable standards
- Directive 2009/105/EC for simple pressure vessels
- European Directive 97/23/EC, EN 13445, EN 13480 on pressure equipment
- Directive 2004/108/EC on electromagnetic compatibility
- EU Directive 2006/95/EC on low voltage electrical equipment
- Machinery Directive 2006/42/EC
Note
Given the required performance, modular design is not possible.
Ammonia (NH 3) is a compound of nitrogen and hydrogen. It is a light gas with a pungent odor. The production of ammonia in industry and laboratories is necessary for the production of fertilizers, polymers, nitric acid and other substances.
In industry
Ammonia is industrially produced from nitrogen by combining it with hydrogen. Nitrogen is taken from the air, hydrogen from water. The method was first developed by the German chemist Fritz Haber. The industrial method for producing ammonia began to be called the Haber process.
The reaction occurs with a decrease in volume and release of energy in the form of heat:
3H 2 + N 2 → 2NH 3 + Q.
The reaction is reversible, so several conditions must be met. At high pressure and low temperatures, the volume of ammonia produced increases. However, low temperatures slow down the reaction rate, and increasing the temperature increases the rate of the reverse reaction.
The necessary conditions for carrying out the reaction were experimentally found:
- temperature- 500°C;
- pressure- 350 atm;
- catalyst- iron oxide Fe 3 O 4 (magnetite) with admixtures of oxides of silver, potassium, calcium and other substances.
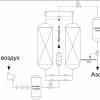
Under these conditions, the resulting gas contains 30% ammonia. To avoid a reverse reaction, the substance is quickly cooled. At low temperatures, the resulting gas turns into a liquid. Unused gases - nitrogen and hydrogen - are returned back to the synthesis column. This method helps to quickly obtain large volumes of ammonia, using raw materials as much as possible.

Rice. 1. Production of ammonia industrially.
To find the right catalyst, 20 thousand different substances were tried.
In the laboratory
To produce ammonia in the laboratory, the reaction of alkalis with ammonium salts is used:
NH 4 Cl + NaOH → NH 3 + NaCl + H 2 O
Ammonia can also be obtained in the laboratory from ammonium chloride heated together with slaked lime, or by decomposition of ammonium hydroxide:
- 2NH 4 Cl + Ca(OH) 2 → CaCl 2 + 2NH 3 + 2H 2 O;
- NH 4 OH ↔ NH 3 + H 2 O.

Rice. 2. Obtaining ammonia in the laboratory.
Ammonia can be completely dried using a mixture of lime and sodium hydroxide, through which the resulting gas is passed. For the same purpose, liquid ammonia is mixed with sodium metal and subjected to distillation.
Ammonia is lighter than air, so to collect it, the test tube is held upside down.
Application
Ammonia is used in various industries:
- in agriculture - for the production of nitrogen-containing fertilizers;
- in industry - for the production of polymers, explosives, artificial ice;
- in chemistry - for the production of nitric acid, soda;
- in medicine - as ammonia.

Rice. 3. Fertilizer production.
What have we learned?
Ammonia is produced industrially and in the laboratory. For production on an industrial scale, nitrogen and hydrogen are used. Mixing under high temperature, pressure and under the influence of a catalyst, simple substances form ammonia. To prevent the reaction from going in the opposite direction at high temperatures, the gas is cooled. In the laboratory, ammonia is obtained by reacting ammonium salts with alkalis, slaked lime, or by decomposing ammonium hydroxide. Ammonia is used in the chemical industry, agriculture, medicine, and chemistry.
Nitrogen is a chemical element that is known to everyone. It is designated by the letter N. It can be said to be the basis of inorganic chemistry, and therefore it begins to be studied in the eighth grade. In this article we will take a closer look at nitrogen, as well as its characteristics and properties.
History of element discovery
Compounds such as ammonia, nitrate, and nitric acid were known and used in practice long before pure nitrogen was obtained in a free state.

In an experiment conducted in 1772, Daniel Rutherford burned phosphorus and other substances in a glass bell. He found that the gas remaining after the combustion of compounds does not support combustion and respiration, and called it “suffocating air.”
In 1787, Antoine Lavoisier established that the gases that make up ordinary air are simple chemical elements, and proposed the name “Nitrogen”. A little later (in 1784), physicist Henry Cavendish proved that this substance is part of nitrate (a group of nitrates). This is where the Latin name for nitrogen comes from (from the Late Latin nitrum and Greek gennao), proposed by J. A. Chaptal in 1790.
By the beginning of the 19th century, scientists had clarified the chemical inertness of the element in a free state and its exceptional role in compounds with other substances. From that moment on, the “binding” of air nitrogen became the most important technical problem in chemistry.
Physical properties

Nitrogen is slightly lighter than air. Its density is 1.2506 kg/m³ (0 °C, 760 mm Hg), melting point - -209.86 °C, boiling point - -195.8 °C. Nitrogen is difficult to liquefy. Its critical temperature is relatively low (-147.1 °C), while the critical pressure is quite high - 3.39 Mn/m². Density in liquid state - 808 kg/m³. This element is less soluble in water than oxygen: 23.3 g of N can be dissolved in 1 m³ (at 0 °C) of H₂O. This figure is higher when working with some hydrocarbons.
When heated to low temperatures, this element interacts only with active metals. For example, with lithium, calcium, magnesium. Nitrogen reacts with most other substances in the presence of catalysts and/or at high temperatures.
The compounds of N with O₂ (oxygen) N₂O₅, NO, N₂O₃, N₂O, NO₂ have been well studied. From them, during the interaction of elements (t - 4000 ° C), NO oxide is formed. Further, during the cooling process, it is oxidized to NO₂. Nitrogen oxides are formed in the air during the passage of atmospheric discharges. They can be obtained by the action of ionizing radiation on a mixture of N and O₂.

When N₂O₃ and N₂O₅ are dissolved in water, respectively, the acids HNO₂ and HNO₂ are obtained, forming salts - nitrates and nitrites. Nitrogen combines with hydrogen exclusively in the presence of catalysts and at high temperatures, forming NH₃ (ammonia). In addition, other (they are quite numerous) compounds of N with H₂ are known, for example diimide HN = NH, hydrazine H₂N-NH₂, octazone N₈H₁₄, acid HN₃ and others.
It is worth saying that most hydrogen + nitrogen compounds are isolated exclusively in the form of organic derivatives. This element does not react (directly) with halogens, so all its halides are obtained only indirectly. For example, NF₃ is formed when ammonia reacts with fluorine.
Most nitrogen halides are weakly stable compounds; oxyhalides are more stable: NOBr, NO₂F, NOF, NOCl, NO₂Cl. Direct combination of N with sulfur also does not occur; N₄S₄ is obtained during the reaction of ammonia + liquid sulfur. When hot coke reacts with N, cyanogen (CN)₂ is formed. By heating acetylene C₂H₂ with nitrogen to 1500 °C, hydrogen cyanide HCN can be obtained. When N interacts with metals at relatively high temperatures, nitrides are formed (for example, Mg₃N₂).
When ordinary nitrogen is exposed to electric discharges [at a pressure of 130–270 n/m² (corresponding to 1–2 mm Hg)] and during the decomposition of Mg₃N₂, BN, TiNx and Ca₃N₂, as well as during electric discharges in the air, active nitrogen can be formed, having increased energy reserves. It, unlike the molecular one, interacts very energetically with hydrogen, sulfur vapor, oxygen, some metals and phosphorus.
Nitrogen is part of quite a few important organic compounds, including amino acids, amines, nitro compounds and others.
Getting nitrogen
In the laboratory, this element can be easily obtained by heating a concentrated solution of ammonium nitrite (formula: NH₄NO₂ = N₂ + 2H₂O). The technical method for obtaining N is based on the separation of pre-liquefied air, which is subsequently subjected to distillation.
Scope of application

The main part of the free nitrogen obtained is used in the industrial production of ammonia, which is then processed in fairly large quantities into fertilizers, explosives, etc.
In addition to the direct synthesis of NH₃ from elements, the cyanamide method developed at the beginning of the last century is used. It is based on the fact that at t = 1000 °C calcium carbide (formed by heating a mixture of coal and lime in an electric furnace) reacts with free nitrogen (formula: CaC₂ + N₂ = CaCN₂ + C). The resulting calcium cyanamide decomposes under the influence of heated water vapor into CaCO₃ and 2NH₃.
In its free form, this element is used in many industries: as an inert medium in various metallurgical and chemical processes, when pumping flammable liquids, for filling space in mercury thermometers, etc. In its liquid state, it is used in various refrigeration units. It is transported and stored in steel Dewar vessels, and compressed gas is stored in cylinders.
Many nitrogen compounds are also widely used. Their production began to develop rapidly after the First World War and has now reached truly enormous proportions.

This substance is one of the main biogenic elements and is part of the most important elements of living cells - nucleic acids and proteins. However, the amount of nitrogen in living organisms is small (approximately 1–3% by dry weight). The molecular material present in the atmosphere is assimilated only by blue-green algae and some microorganisms.
Quite large reserves of this substance are concentrated in the soil in the form of various mineral (nitrates, ammonium salts) and organic compounds (composed of nucleic acids, proteins and their breakdown products, including not yet completely decomposed remains of flora and fauna).
Plants perfectly absorb nitrogen from the soil in the form of organic and inorganic compounds. Under natural conditions, special soil microorganisms (ammonifiers) are of great importance, which are capable of mineralizing soil organic N to ammonium salts.
Nitrate nitrogen in the soil is formed during the life of nitrifying bacteria, discovered by S. Winogradsky in 1890. They oxidize ammonium salts and ammonia to nitrates. Part of the substance assimilated by flora and fauna is lost due to the action of denitrifying bacteria.
Microorganisms and plants perfectly absorb both nitrate and ammonium N. They actively convert inorganic material into various organic compounds - amino acids and amides (glutamine and asparagine). The latter are part of many proteins of microorganisms, plants and animals. The synthesis of asparagine and glutamine by amidation (enzymatic) of aspartic and glutamic acids is carried out by many representatives of flora and fauna.
The production of amino acids occurs through the reductive amination of a number of keto acids and aldehyde acids, resulting from enzymatic transamination, as well as as a result of the oxidation of various carbohydrates. The end products of ammonia (NH₃) assimilation by plants and microorganisms are proteins, which are part of the cell nucleus, protoplasm, and are also deposited in the form of so-called storage proteins.
Humans and most animals can synthesize amino acids only to a fairly limited extent. They are not able to produce eight essential compounds (lysine, valine, phenylalanine, tryptophan, isoleucine, leucine, methionine, threonine), and therefore their main source of nitrogen is proteins consumed with food, that is, ultimately, the own proteins of microorganisms and plants.