Polymer - what is it? Polymer production. History of polymers Which polymer is of natural origin
From the history of polymers
The first mentions of synthetic polymers date back to 1838 (polyvinylidene chloride) and 1839 (polystyrene). A number of polymers may have been prepared as early as the first half of the 19th century. But in those days, chemists tried to suppress polymerization and polycondensation, which led to the “resinization” of the products of the main chemical reaction, that is, to the formation of polymers (polymers are still often called “resins”).
In 1833, I. Berzelius first used the term “polymerism” to designate a special type of isomerism. In this isomerism, substances (polymers) having the same composition had different molecular weights, for example, ethylene and butylene, oxygen and ozone. However, that term had a slightly different meaning than modern ideas about polymers. “True” synthetic polymers were not yet known at that time.
A. M. Butlerov studied the relationship between the structure and relative stability of molecules, manifested in polymerization reactions. After A. M. Butlerov created the theory of chemical structure, polymer chemistry arose. The science of polymers was developed mainly due to the intensive search for methods of synthesizing rubber. Scientists from many countries took part in these studies, such as G. Bouchard, W. Tilden, German scientist K. Harries, I. L. Kondakov, S. V. Lebedev and others. The works of W. Carothers played a major role in the development of ideas about polycondensation.
In the 30s, the existence of free radical and ionic polymerization mechanisms was proven.
Since the beginning of the 20s of the 20th century, G. Staudinger became the author of a fundamentally new concept of polymers as substances consisting of macromolecules, particles of unusually large molecular weight. Previously, it was assumed that biopolymers such as cellulose, starch, rubber, proteins, as well as some synthetic polymers with similar properties (for example, polyisoprene), consist of small molecules that have an unusual ability to associate in solution into complexes of a colloidal nature due to non-covalent bonds (the theory of “small blocks”). However, G. Staudinger's discovery forced us to consider polymers as a qualitatively new object of study in chemistry and physics.
Polymers are chemical compounds with high molecular weight (from several thousand to many millions), whose molecules (macromolecules) consist of a large number of repeating groups (monomer units). The atoms that make up macromolecules are connected to each other by forces of principal and (or) coordination valences.
Classification of polymers
Polymers can be classified according to their origin. They are divided into natural (biopolymers) and synthetic. Biopolymers include proteins, nucleic acids, natural resins, and synthetic polymers include polyethylene, polypropylene, phenol-formaldehyde resins.
Polymers are also classified according to the arrangement of atoms in the macromolecule. Atoms or atomic groups can be located in a macromolecule in the form:
- an open chain or an elongated sequence of cycles (linear polymers, for example natural rubber);
- branched chains (branched polymers, e.g. amylopectin), three-dimensional networks (cross-linked polymers, e.g. cured epoxy resins).
Polymers whose molecules consist of identical monomer units are called homopolymers (these include polyvinyl chloride, polycaproamide, cellulose).
Polymers whose macromolecules contain several types of monomer units are called copolymers. Copolymers in which units of each type form sufficiently long continuous sequences that replace each other within the macromolecule are called block copolymers. One or more chains of another structure can be attached to the internal (non-terminal) links of a macromolecule of one chemical structure. Such copolymers are called graft copolymers.
Macromolecules of the same chemical composition can be built from units of different spatial configurations. If macromolecules consist of the same stereoisomers or of different stereoisomers alternating in the chain at a certain periodicity, the polymers are called stereoregular.
Based on the composition of the main (main) chain, polymers are divided into: heterochain, the main chain of which contains atoms of various elements, most often carbon, nitrogen, silicon, phosphorus, and homochain, the main chain of which is built from identical atoms.
Polymers in which each or some stereoisomers of a unit form sufficiently long continuous sequences that replace each other within one macromolecule are called stereoblock copolymers.
Of the homochain polymers, the most common are carbon chain polymers, the main chains of which consist only of carbon atoms, for example, polyethylene, polymethyl methacrylate, polytetrafluoroethylene.
Examples of heterochain polymers are polyesters (polyethylene terephthalate, polycarbonates), polyamides, urea-formaldehyde resins, proteins, and some organosilicon polymers. Polymers whose macromolecules, along with hydrocarbon groups, contain atoms of inorganogenic elements are called organoelement.
A separate group of polymers is formed by inorganic polymers, for example, plastic sulfur, polyphosphonitrile chloride.
Properties and main characteristics of polymers
Polymers can exist in crystalline and amorphous states. A necessary condition for crystallization is the regularity of sufficiently long sections of the macromolecule. In crystalline polymers, various supramolecular structures can arise: fibrils, spherulites, single crystals, the type of which largely determines the properties of the polymer material. Supramolecular structures in non-crystallized (amorphous) polymers are less pronounced than in crystalline ones.
Cellulose, a polymer with very rigid chains connected by intermolecular hydrogen bonds, generally cannot exist in a highly elastic state before its decomposition temperature. Large differences in the properties of polymers can be observed even if the differences in the structure of macromolecules are, at first glance, small. Thus, stereoregular polystyrene is a crystalline substance with a melting point of about 235 °C, while non-stereoregular polystyrene is not able to crystallize at all, and softens at a temperature of about 80 °C.
Non-crystallized polymers can be in three physical states: glassy, highly elastic and viscous-fluid. Polymers with a low (below room) temperature of transition from a glassy to a highly elastic state are called elastomers, while those with a high temperature are called plastics. Depending on the chemical composition, structure and relative arrangement of macromolecules, the properties of polymers can vary within very wide limits. Thus, 1,4.-cispolybutadiene, built from flexible hydrocarbon chains, at a temperature of about 20 ° C is an elastic material, which at a temperature of -60 ° C transforms into a glassy state; polymethyl methacrylate, built from more rigid chains, at a temperature of about 20 ° C is a solid glassy product that turns into a highly elastic state only at 100 ° C.
Linear polymers have a specific set of physicochemical and mechanical properties. The most important of these properties:
- ability to form high-strength anisotropic highly oriented fibers and films , ability to large, long-term reversible deformations;
- the ability to swell in a highly elastic state before dissolving;
- high viscosity of solutions.
This set of properties is due to the high molecular weight, chain structure, and flexibility of macromolecules. When moving from linear chains to branched, sparse three-dimensional networks and, finally, to dense mesh structures, this set of properties becomes less and less pronounced. Highly cross-linked polymers are insoluble, infusible, and incapable of highly elastic deformations.
Polymers can undergo the following main types of reactions:
- the formation of chemical bonds between macromolecules (so-called cross-linking), for example, during vulcanization of rubbers, tanning of leather;
- decomposition of macromolecules into separate, shorter fragments, reactions of side functional groups of polymers with low-molecular substances that do not affect the main chain (so-called polymer-analogous transformations);
- intramolecular reactions occurring between functional groups of one macromolecule, for example, intramolecular cyclization. Cross-linking often occurs simultaneously with destruction.
An example of polymer-analogous transformations is the saponification of polytyl acetate, leading to the formation of polyvinyl alcohol. The rate of reactions of polymers with low molecular weight substances is often limited by the rate of diffusion of the latter into the polymer phase. This is most obvious in the case of cross-linked polymers. The rate of interaction of macromolecules with low-molecular substances often significantly depends on the nature and location of neighboring units relative to the reacting unit. The same applies to intramolecular reactions between functional groups belonging to the same chain.
Some properties of polymers, for example, solubility, ability to viscous flow, stability, are very sensitive to the action of small amounts of impurities or additives that react with macromolecules. Thus, to transform a linear polymer from soluble to completely insoluble, it is enough to form 1–2 cross-links per macromolecule.
The most important characteristics of polymers are their chemical composition, molecular weight and molecular weight distribution, the degree of branching and flexibility of macromolecules, stereoregularity, and others.
Preparation of polymers
Natural polymers are formed during the process of biosynthesis in the cells of living organisms. Using extraction, fractional precipitation and other methods, they can be isolated from plant and animal materials. Synthetic polymers are produced by polymerization and polycondensation. Carbochain polymers are usually synthesized by polymerization of monomers with one or more multiple carbon bonds or monomers containing unstable carbocyclic groups (for example, from cyclopropane and its derivatives). Heterochain polymers are obtained by polycondensation, as well as polymerization of monomers containing multiple bonds of a carbon element (for example, C = O, C = N, N = C = O) or weak heterocyclic groups.
Polymers in agriculture
Today we can talk about at least four main areas of using polymer materials in agriculture. Both in domestic and in world practice, the first place belongs to films. Thanks to the use of perforated mulching film in the fields, the yield of some crops increases up to 30%, and the ripening time is accelerated by 10 - 14 days. The use of polyethylene film for waterproofing created reservoirs ensures a significant reduction in losses of stored moisture. Covering haylage, silage, and roughage with film ensures their better preservation even in adverse weather conditions. But the main area of use of film polymer materials in agriculture is the construction and operation of film greenhouses. Currently, it has become technically possible to produce sheets of film up to 16 m wide, and this makes it possible to build film greenhouses with a base width of up to 7.5 and a length of up to 200 m. In such greenhouses, all agricultural work can be carried out mechanized; Moreover, these greenhouses allow you to grow produce all year round. In cold weather, greenhouses are heated again using polymer pipes buried in the soil to a depth of 60–70 cm.
From the point of view of the chemical structure of polymers used in greenhouses of this kind, one can note the predominant use of polyethylene, unplasticized polyvinyl chloride and, to a lesser extent, polyamides. Polyethylene films are characterized by better light transmission, better strength properties, but poorer weather resistance and relatively high heat loss. They can only serve properly for 1 – 2 seasons. Polyamide and other films are still used relatively rarely.
Job Description
The first mentions of synthetic polymers date back to 1838 (polyvinylidene chloride) and 1839 (polystyrene). A number of polymers may have been prepared as early as the first half of the 19th century. But in those days, chemists tried to suppress polymerization and polycondensation, which led to the “resinization” of the products of the main chemical reaction, that is, to the formation of polymers (polymers are still often called “resins”).
Most of modern building materials, medicines, fabrics, household items, packaging and consumables are polymers. This is a whole group of compounds that have characteristic distinctive features. There are a lot of them, but despite this, the number of polymers continues to grow. After all, synthetic chemists discover more and more new substances every year. At the same time, it was the natural polymer that was of particular importance at all times. What are these amazing molecules? What are their properties and what are their features? We will answer these questions during the article.
Polymers: general characteristics
From a chemical point of view, a polymer is considered to be a molecule with a huge molecular weight: from several thousand to millions of units. However, in addition to this characteristic, there are several more by which substances can be classified specifically as natural and synthetic polymers. This:
- constantly repeating monomer units that are connected through various interactions;
- the degree of polymerization (that is, the number of monomers) must be very high, otherwise the compound will be considered an oligomer;
- a certain spatial orientation of the macromolecule;
- a set of important physicochemical properties characteristic only of this group.
In general, a substance of a polymeric nature is quite easy to distinguish from others. One only has to look at its formula to understand this. A typical example is the well-known polyethylene, widely used in everyday life and industry. It is a product into which ethene or ethylene enters. The reaction in general form is written as follows:
nCH 2 =CH 2 → (-CH-CH-) n, where n is the degree of polymerization of the molecules, indicating how many monomer units are included in its composition.
Also, as an example, we can cite a natural polymer that is well known to everyone, this is starch. In addition, amylopectin, cellulose, chicken protein and many other substances belong to this group of compounds.
Reactions that can result in the formation of macromolecules are of two types:
- polymerization;
- polycondensation
The difference is that in the second case the reaction products are low molecular weight. The structure of a polymer can be different, it depends on the atoms that form it. Linear forms are common, but there are also three-dimensional mesh forms that are very complex.
If we talk about the forces and interactions that hold monomer units together, we can identify several main ones:
- Van Der Waals forces;
- chemical bonds (covalent, ionic);
- Electronostatic interaction.
All polymers cannot be combined into one category, since they have completely different natures, methods of formation and perform different functions. Their properties also differ. Therefore, there is a classification that allows you to divide all representatives of this group of substances into different categories. It may be based on several signs.

Classification of polymers
If we take the qualitative composition of molecules as a basis, then all the substances under consideration can be divided into three groups.
- Organic are those that contain atoms of carbon, hydrogen, sulfur, oxygen, phosphorus, and nitrogen. That is, those elements that are biogenic. There are a lot of examples: polyethylene, polyvinyl chloride, polypropylene, viscose, nylon, natural polymer - protein, nucleic acids, and so on.
- Organic elements are those that contain some foreign inorganic and non-organic element. Most often it is silicon, aluminum or titanium. Examples of such macromolecules: glass polymers, composite materials.
- Inorganic - the chain is based on silicon atoms, not carbon. Radicals can also be part of side branches. They were discovered quite recently, in the middle of the 20th century. Used in medicine, construction, technology and other industries. Examples: silicone, cinnabar.
If we divide polymers by origin, we can distinguish three groups.
- Natural polymers, the use of which has been widely carried out since ancient times. These are macromolecules for which man did not make any effort to create. They are products of reactions of nature itself. Examples: silk, wool, protein, nucleic acids, starch, cellulose, leather, cotton and others.
- Artificial. These are macromolecules that are created by humans, but based on natural analogues. That is, the properties of an existing natural polymer are simply improved and changed. Examples: artificial
- Synthetic polymers are those in which only humans are involved in their creation. There are no natural analogues for them. Scientists are developing methods for synthesizing new materials that would have improved technical characteristics. This is how synthetic polymer compounds of various kinds are born. Examples: polyethylene, polypropylene, viscose, etc.
There is one more feature that underlies the division of the substances under consideration into groups. These are reactivity and thermal stability. There are two categories for this parameter:
- thermoplastic;
- thermosetting.
The most ancient, important and especially valuable is still a natural polymer. Its properties are unique. Therefore, we will further consider this category of macromolecules.

What substance is a natural polymer?
To answer this question, let's first look around us. What surrounds us? Living organisms around us that eat, breathe, reproduce, bloom and produce fruits and seeds. What are they from a molecular point of view? These are connections such as:
- proteins;
- nucleic acids;
- polysaccharides.
So, each of the above compounds is a natural polymer. Thus, it turns out that life around us exists only due to the presence of these molecules. Since ancient times, people have used clay, building mixtures and mortars to strengthen and create homes, weave yarn from wool, and use cotton, silk, wool and animal skin to create clothing. Natural organic polymers accompanied man at all stages of his formation and development and largely helped him achieve the results that we have today.
Nature itself gave everything to make people’s lives as comfortable as possible. Over time, rubber was discovered and its remarkable properties were discovered. Man learned to use starch for food purposes and cellulose for technical purposes. Camphor, which has also been known since ancient times, is a natural polymer. Resins, proteins, nucleic acids are all examples of compounds considered.
Structure of natural polymers
Not all representatives of this class of substances are structured the same. Thus, natural and synthetic polymers can differ significantly. Their molecules are oriented in such a way that they exist as advantageously and conveniently as possible from an energetic point of view. At the same time, many natural species are capable of swelling and their structure changes in the process. There are several most common variants of the chain structure:
- linear;
- branched;
- star-shaped;
- flat;
- mesh;
- tape;
- comb-shaped.
Artificial and synthetic representatives of macromolecules have a very large mass and a huge number of atoms. They are created with specially specified properties. Therefore, their structure was initially planned by man. Natural polymers are most often either linear or network in structure.

Examples of natural macromolecules
Natural and artificial polymers are very close to each other. After all, the former become the basis for creating the latter. There are many examples of such transformations. Let's list some of them.
- Conventional milky-white plastic is a product obtained by treating cellulose with nitric acid with the addition of natural camphor. The polymerization reaction causes the resulting polymer to harden and become the desired product. And the plasticizer, camphor, makes it capable of softening when heated and changing its shape.
- Acetate silk, copper-ammonia fiber, viscose - all these are examples of those threads and fibers that are obtained from cellulose. Fabrics made from linen are not so durable, not shiny, and easily wrinkled. But artificial analogues do not have these disadvantages, which makes their use very attractive.
- Artificial stones, building materials, mixtures, leather substitutes are also examples of polymers obtained from natural raw materials.
The substance, which is a natural polymer, can be used in its true form. There are also many such examples:
- rosin;
- amber;
- starch;
- amylopectin;
- cellulose;
- wool;
- cotton;
- silk;
- cement;
- clay;
- lime;
- proteins;
- nucleic acids and so on.
It is obvious that the class of compounds we are considering is very numerous, practically important and significant for people. Now let's take a closer look at several representatives of natural polymers that are in great demand at the moment.

Silk and wool
The formula of natural silk polymer is complex, because its chemical composition is expressed by the following components:
- fibroin;
- sericin;
- waxes;
- fats.
The main protein itself, fibroin, contains several types of amino acids. If you imagine its polypeptide chain, it will look something like this: (-NH-CH 2 -CO-NH-CH(CH 3)-CO-NH-CH 2 -CO-) n. And this is just part of it. If we imagine that an equally complex sericin protein molecule is attached to this structure with the help of Van Der Waals forces, and together they are mixed into a single conformation with wax and fats, then it is clear why it is difficult to depict the formula of natural silk.
Today, most of this product is supplied by China, because in its vastness there is a natural habitat for the main producer - the silkworm. Previously, since ancient times, natural silk was highly valued. Only noble, rich people could afford clothes made from it. Today, many characteristics of this fabric leave much to be desired. For example, it becomes strongly magnetized and wrinkles; in addition, it loses its luster and becomes dull when exposed to the sun. Therefore, artificial derivatives based on it are more common.
Wool is also a natural polymer, as it is a waste product of the skin and sebaceous glands of animals. Based on this protein product, knitwear is made, which, like silk, is a valuable material.

Starch
The natural polymer starch is a waste product of plants. They produce it through the process of photosynthesis and accumulate it in different parts of the body. Its chemical composition:
- amylopectin;
- amylose;
- alpha glucose.
The spatial structure of starch is very branched and disordered. Thanks to the amylopectin it contains, it is able to swell in water, turning into a so-called paste. This one is used in engineering and industry. Medicine, the food industry, and the production of wallpaper adhesives are also areas of use of this substance.
Among the plants containing the maximum amount of starch are:
- corn;
- potato;
- wheat;
- cassava;
- oats;
- buckwheat;
- bananas;
- sorghum.
Based on this biopolymer, bread is baked, pasta is made, jelly, porridge and other food products are cooked.

Cellulose
From a chemical point of view, this substance is a polymer, the composition of which is expressed by the formula (C 6 H 5 O 5) n. The monomeric unit of the chain is beta-glucose. The main places where cellulose is contained are the cell walls of plants. That is why wood is a valuable source of this compound.
Cellulose is a natural polymer that has a linear spatial structure. It is used to produce the following types of products:
- pulp and paper products;
- faux fur;
- different types of artificial fibers;
- cotton;
- plastics;
- smokeless powder;
- films and so on.
It is obvious that its industrial significance is great. In order for this compound to be used in production, it must first be extracted from plants. This is done by long-term cooking of wood in special devices. Further processing, as well as the reagents used for digestion, vary. There are several ways:
- sulfite;
- nitrate;
- soda;
- sulfate.
After this treatment, the product still contains impurities. It is based on lignin and hemicellulose. To get rid of them, the mass is treated with chlorine or alkali.
There are no biological catalysts in the human body that would be able to break down this complex biopolymer. However, some animals (herbivores) have adapted to this. Certain bacteria settle in their stomach and do this for them. In return, microorganisms receive energy for life and a habitat. This form of symbiosis is extremely beneficial for both parties.

Rubber
It is a natural polymer of valuable economic importance. It was first described by Robert Cook, who discovered it on one of his travels. It happened like this. Having landed on an island where natives unknown to him lived, he was hospitably received by them. His attention was attracted by local children who were playing with an unusual object. This spherical body pushed off from the floor and jumped high up, then returned.
Having asked the local population what this toy was made of, Cook learned that this is how the sap of one of the trees, the Hevea, solidifies. Much later it was found out that this is the biopolymer rubber.
The chemical nature of this compound is known - it is isoprene that has undergone natural polymerization. Rubber formula (C 5 H 8) n. Its properties, due to which it is so highly valued, are as follows:
- elasticity;
- wear resistance;
- electrical insulation;
- waterproof.
However, there are also disadvantages. In the cold it becomes brittle and brittle, and in the heat it becomes sticky and viscous. That is why there was a need to synthesize analogues of an artificial or synthetic base. Today, rubbers are widely used for technical and industrial purposes. The most important products based on them:
- rubber;
- ebony.
Amber
It is a natural polymer, since its structure is a resin, its fossil form. The spatial structure is a framework amorphous polymer. It is very flammable and can be ignited with a match flame. Has luminescent properties. This is a very important and valuable quality that is used in jewelry. Amber-based jewelry is very beautiful and in demand.
In addition, this biopolymer is also used for medical purposes. Sandpaper and varnish coatings for various surfaces are also made from it.
INTRODUCTION
HISTORY OF THE DEVELOPMENT OF THE PROCESSING INDUSTRY
POLYMERS
Polymer processing appeared in the mid-19th century
Modified cellulose - celluloid - for replacement
elephant tusks for billiard balls.
Devices called extruders appeared in the 19th century in
England, America and Germany. Used for insulation
wires and cables with rubber.
3
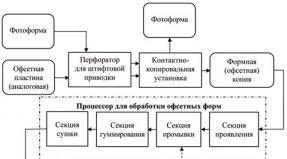
Chemical industry branch: “Synthetic resins and their processing.”
1. CURRENT STATE OF THE INDUSTRYPROCESSING OF POLYMERS AND COMPOSITES
Chemical industry sector:
"Synthetic resins and their processing."
Raw materials
Recycling:
Generates maximum
number of seats
Works like a driver
mechanical engineering development
Increases knowledge intensity
industry of the country
Polymer
Recycling
45
Prerequisites for the development of polymer processing
Annualpace
development
industrial
production
Russia
8,2%
4,7%
3,3%
0,3%
3,2%
5%
Annual
pace
development
processing
plastics
21,5%
13,1%
7,4%
7,3%
7-7,5%
9-10%
realistic
2010
2011
2012
2013
optimistic
2030
672. CLASSIFICATION OF POLYMER MATERIALS
AND COMPOSITES
1. Classification by modulus of elasticity under conditions
operation
At room temperature, the elastic modulus at
stretching of polymers in a highly elastic state is very
small
E~ 0.1 - 10 MPa
For glassy polymers - plastics - E 103 MPa
For partially crystalline polymers - plastics
E (αcrist) 10 – 103 MPa
2. Classification according to glass transition temperature of amorphous polymers
Tst< Т = 23оС < Тст
elastomer
plastic
8Polymers that, under operating conditions
are in glassy or crystalline
states and exhibit elasticity, are used in
quality of construction materials and are
basis of plastics and fibers.
Polymers that, under operating conditions
are in a highly elastic physical state
and exhibit large and reversible deformations,
used as elastomers.
9
10.
1011.
3. Classification by chemical structure11
12.
4. Classification according to technological characteristics:Thermoplastics - PM, capable of repeated
transition when heated to a fluid state and
solidification upon cooling without significant
changes in structure and properties.
Thermoset plastics are PM, which when heated at first
go into a fluid state and then harden
as a result of chemical transformations and are incapable of
re-transition to a fluid state.
4. CLASSIFICATION according to technological properties (conditional)
character):
injection molded (for thin-walled products, long
products),
extrusion (film, pipe, sheet),
press materials.
12
13.
5. Classification by areas of application - identification of groupsPMs that are similar in their main operational characteristics.
Construction materials – for work under short-term or
long-term exposure to static loads: E > 900 MPa
(PA, PC, PBT, PFO, polyimides, etrols, reinforced PP, PA, phenolic plastics,
aminoplasts, organosilicon compositions).
Impact-resistant materials - work under shock loads: shock
strength > 20 kJ/m2
(PE, EPDM, SEVA, PP, PVC, PTFE, UPS, PC, ABS, reinforced plastics).
Heat-resistant materials – operating temperature > 150°C
(PA, PBTF, PET, PFO, PC, aminoplasts, phenoplasts, polyimides, rubber-based
fluorine rubbers, organosilicon compositions)
Frost-resistant materials - operating conditions< минус 40оС
(PE, EPDM, SEVA, PTFE, PA, PC, rubber based on NC, isoprene, etc.).
PM for electrical and radio engineering purposes – ρv > 1010 Ohm*m, tg δ< 0,02
(PO, PVC, PTFE, PPS, PFO, polyimides, SFD, unsaturated PE).
13
14.
Lighting PM – light transmittance > 80%(block PS, SAN copolymers, polyacrylates, PMMA, transparent grades PVC, PC,
films PET and PA, ES, unsaturated PE)
Fire-resistant and self-extinguishing PM - CI > 22% or attenuated when removed
from the flames
(PTFE, polyimides, PVC and compositions with flame retardants)
Radiation-resistant PM – long-term resistance to ionizing
radiation
(PTFE, polymides, fluorine rubbers, compositions based on ES and KS).
Chemically resistant PM - for work in aggressive environments
(PO, PVC, PTFE, PBT, PET, polyimides, compositions based on CS).
In addition, groups of water-, petrol-, oil-resistant, weather-resistant,
tropical-resistant, fungus-resistant PM.
14
15.
6. Classification of PM according to a set of operating parameters15
16.
7. Classification of PM by production volumeLarge-scale plastics: PO, PVC, PS and its copolymers, PU,
compositions based on FPS, unsaturated PES, aminoaldehyde resins
– 80% of total plastics production.
Large-capacity rubbers – general purpose rubbers: isoprene,
butadiene, styrene butadiene.
Medium-tonnage plastics: PA, etrols, PET, PC, PFO, ES,
furan resins.
Medium-tonnage rubbers – chloroprene, acrylate,
ethylene propylene.
Small-scale plastics and rubbers – a few% of volume
production.
16
17.
8. Classification by composition and type of macrostructurepolymer material:
Polymer material (PM) = polymer + ∑ additives
Homogeneous macrostructure: all additives (stabilizers,
plasticizers, dyes) are dissolved in the polymer.
Heterogeneous macrostructure - composite materials:
additives are insoluble in the polymer - presence of inclusions
(fillers, pigments, polymer additives) size
more than 100 nm.
The heterogeneous structure of the PM can be:
17
18.
1819.
Types of reinforced structures19
20.
3. CREATION OF POLYMER MATERIALSPOLYMERIC
MATERIAL
POLYMER
POLYMERIC
MATERIAL
=
POLYMER + Σ ADDITIVES
ADDITIVES:
1 ANTI-AGING POLYMER MATERIALS
2 IMPROVING PROCESSABILITY
3 REDUCE FLAMMABILITY
4 INCREASING STRENGTH PROPERTIES
5 GIVING SPECIAL PROPERTIES
20
21.
1 ADDITIVES THAT SLOW AGING PMAging of polymers is a complex complex of chemical and
physical processes occurring under the influence of the environment
environment during PM processing, operation and storage,
leading to irreversible or reversible changes
(deterioration) of the properties of polymers (instead of the term “aging”
They use the term “destruction” (sometimes “degradation”).
The processes of physical aging are reversible. No gap or
cross-linking of polymer chains. These are crystallization processes
recrystallization or diffusion of solvents into the polymer,
causing intergranular corrosion and leading to
deterioration of the mechanical properties of polymer products.
21
22.
Chemical aging processes are irreversible. Theylead to the breaking of chemical bonds, and sometimes to cross-linking
macromolecules, changes in chemical structure, decrease
or increasing the molecular weight of the polymer.
Chemical aging processes:
Thermal destruction (thermal decomposition of macromolecules by
chain mechanism).
Oxidative destruction (formation of peroxide
radicals that initiate chain decomposition).
As a rule, TD and OD go simultaneously - this is
thermal-oxidative destruction.
Ozone aging (ozone reacts with a double bond,
an intermediate complex arises and then forms
cyclic oxygen-containing compound that decomposes
to peroxide radicals)
22
23.
PhotodestructionPhotochemical transformations occur in
polymers under the influence of UV (180< λ< 400нм) и
visible light (400< λ < 800нм), если полимер
contains chemical bonds or chromophores
groups.
Photodestruction in this area is subject to
polymers containing
O, N, double bonds,
aromatic nuclei, impurities of metal compounds
(for example, catalyst residues) accidentally ingested
aromatic compounds, etc.
23
24.
Radiation destruction. Ionizing radiation leadsto the formation of an electron and a positively charged polymer
particles that break down into radicals.
Hydrolytic destruction. Process mechanism
hydrolytic, the rate of destruction is determined by the speed
diffusion of hydrolytic medium (water, acid solutions,
bases, salts).
Mechanical destruction. The formation of radicals under the influence
mechanical forces with subsequent transformation in air into
hydroperoxides.
Biodegradation. Interaction with bacteria, fungi and
by hydrolytic enzymatic decomposition
polymer.
24
25.
2526.
2627.
STABILIZERS (thermal and light stabilizers, antioxidants,antirads, antiozonates, fungicides).
Thermal stabilizers (or inhibitors):
a) terminate chains by reaction with peroxide radicals
(phenols, aromatic amines, aminophenols, hydroxylamines,
aromatic polynuclear hydrocarbons).
b) terminate chains by reaction with alkyl (R*) radicals
(quinones, nitroxyl radicals, iodine molecules).
c) destroy hydroperoxides, especially in reactions
autoxidation (sulfides, disulfides, phosphorous esters
acid).
27
28.
Light stabilizers:a) substances reflecting light quanta - (technical
carbon (soot)),
b) absorbing light quanta (2-hydroxybenzophenone),
c) quenchers of excited states (2-(2`-hydroxyphenyl)-benzothiazole).
Antioxidants are substances that interact with
peroxides and hydroxides (primary antioxidants -
hindered phenols, secondary phenols - phosphites, thioesters).
Anti-radiation additives (anti-rads) provide resistance to gamma radiation and other types
radiation during sterilization.
Antimicrobial additives, abiotic additives,
biocides. Increases resistance to bacterial action
(bactericides), fungi and mold (fungicides), fouling in water
(algaecides)
28
29.
2930.
3031.
Antioxidant requirements:effective protection of the polymer during the process
processing into products; from external influences
during operation;
stabilizer losses during processing should
be minimal;
fairly high MW (over 700 g/mol);
ability to diffuse in a polymer melt;
compatibility, solubility in solids
polymer;
low volatility and resistance to migration into
environment;
special stabilizers for products
medical purposes, toys, packaging
31
food products
Ministry of Education and Science of the Russian Federation
federal state autonomous educational institution
higher professional education
"NATIONAL RESEARCH
TOMSK POLYTECHNIC UNIVERSITY"
Institute of Natural Resources
Direction of training (specialty) Chemical technology
Department of Chemical Technology of Fuel and Chemical Cybernetics
Abstract
Abstract title:
“ Natural polymers, polymers around us “
in the discipline "Introduction to Engineering"
Completed by students gr. 2D42 Nikonova Nyurguyaana
Prokopchuk Kristina
Dayanova Regina
Abstract accepted:
Moises O. E.
(Signature)
2014
(date of report verification)
Tomsk 2014
1.Introduction…………………………………………………………………………………………..2
2. Concept of polymer and classification ………………………………………………….3
3.Pulp……………………………………………………………………………………………………………3
4. Starch……………………………………………………………………………………………………………5
5. Glutin……………………………………………………………………………………………………………..6
6.Casein……………………………………………………………………………………………………………6
7. Rubber………………………………………………………………………………………………………………….7
8. Rubber……………………………………………………………………………………………………………7
9. Synthetic polymers………………………………………………………………...9
10.Properties and most important characteristics……………………………………………10
11. Chemical reactions………………………………………………………………………………….11
12.Receipt……………………………………………………………………………………………………………12
13.Polymers in agriculture…………………………………………………………..12
14.Polymers in industry…………………………………………………………….14
Introduction
The term “polymerism” was introduced into science by I. Berzelius in 1833 to designate a special type of isomerism, in which substances (polymers) having the same composition have different molecular weights, for example, ethylene and butylene, oxygen and ozone. This content of the term did not correspond to modern ideas about polymers. “True” synthetic polymers were not yet known at that time.
A number of polymers were apparently prepared as early as the first half of the 19th century. However, chemists then usually tried to suppress polymerization and polycondensation, which led to the “resinization” of the products of the main chemical reaction, i.e., in fact, to the formation of polymers (polymers are still often called “resins”). The first mentions of synthetic polymers date back to 1838 (polyvinylidene chloride) and 1839 (polystyrene),
Polymer chemistry arose only in connection with the creation of the theory of chemical structure by A.M. Butlerov. A.M. Butlerov studied the relationship between the structure and relative stability of molecules, manifested in polymerization reactions. The science of polymers received its further development mainly due to the intensive search for methods of synthesizing rubber, in which the leading scientists of many countries participated (G. Buscharda, W. Tilden, German scientist K. Harries, I. L. Kondakov, S. V. Lebedev and others ). In the 30s, the existence of free radical and ionic polymerization mechanisms was proven. The works of W. Carothers played a major role in the development of ideas about polycondensation.
Since the beginning of the 20s of the 20th century, theoretical ideas about the structure of polymers have also been developing. Initially, it was assumed that biopolymers such as cellulose, starch, rubber, proteins, as well as some synthetic polymers similar to them in properties (for example, polyisoprene), consist of small molecules that have the unusual ability to associate in solution into complexes of a colloidal nature due to non-covalent bonds (the theory of “small blocks”). The author of a fundamentally new concept of polymers as substances consisting of macromolecules, particles of unusually large molecular weight, was G. Staudinger. The victory of this scientist’s ideas forced us to consider polymers as a qualitatively new object of study in chemistry and physics.
Polymer concept and classification
Polymers- chemical compounds with high molecular weight (from several thousand to many millions), the molecules of which (macromolecules) consist of a large number of repeating groups (monomer units). The atoms that make up macromolecules are connected to each other by forces of principal and (or) coordination valences.
Classification.
Based on their origin, polymers are divided into:
natural (biopolymers), for example proteins, nucleic acids, natural resins
synthetic, for example polyethylene, polypropylene, phenol-formaldehyde resins.
Natural polymers used in printing include: polysaccharides (cellulose starch, gums), spruce, glutin, casein, albumin), polydienes (rubber).
Cellulose
Cellulose, or fiber (from the Latin word "cellulula" - cell), is widespread in nature. Cellulose is a strong fibrous substance of organic origin that makes up the supporting tissue of all plants (plant cells).
Physical properties of cellulose
Cellulose fibers are distinguished by their whiteness, flexibility, strength, elasticity, i.e. the ability to reversibly deform without destruction even under high mechanical stress, insolubility in water and organic solvents, and infusibility.
Cellulose can withstand heating up to 150° without destruction; at higher temperatures, depolymerization of cellulose and the associated loss of strength are observed, and at 270° and above, thermal decomposition begins with the release of decomposition products: acetic acid, methyl alcohol, ketones, with the remainder being tar and coal.
The structure of cellulose fiber.
Each plant fiber, for example cotton, flax, wood, etc., is one cell, the shell of which consists mainly of cellulose. Inside the fiber there is a channel - a capillary, accessible for the penetration of air and moisture. Technical cellulose fibers have an average length of 2.5-3 mm (spruce, pine, birch, poplar) and 20-25 mm (flax, cotton, hemp) with a diameter of 25 microns.
Cellulose plant fiber has a fibrillar structure. Fibrils are thread-like, elementary roll windows - packs of cellulose molecules, firmly connected to each other by hydrogen bonds, 50 µm long and 0.1-0.4 µm in diameter. Most likely, cellulose forms an ordered system of threads - fibrils, located more tightly around the internal channel (capillary) of the fiber and more loosely in its outer layers. In the spaces between the fibrils there are micelluloses and lignin, and their content increases from the inner layers of the cell structure to the outer ones. The intercellular spaces of cellulose are filled predominantly with lignin.
The main source of cellulose is wood... Wood is the internal part of trees, lying under the bark and constituting the main plant tissue from which the tree trunk is formed.
A living cell of a growing tree has a cellulose shell (walls), an internal cavity filled with protoplasm, and a nucleus. A living cell is capable of growing and forming new wood formations in the cambium layer, under the bark, from year to year in a growing tree.
Living cells undergo lignification over time, ultimately leading to their complete death, or lignification. Cell lignification occurs mainly as a result of the appearance of lignin in it. Wood consists of 90-95% of such dead cells - fibers, devoid of protoplasm and nucleus, but capable of division, with an internal cavity filled with air and water.
Chemical structure and properties of cellulose. Cellulose is a natural polysaccharide polymer belonging to the carbohydrate class. The giant molecule (macromolecule) of cellulose is built from repeatedly repeating structural units - β-glucose residues (O6H10O5)p. The number n, or polymerization coefficient, shows how many times the structural unit - the β-glucose residue - is repeated in the cellulose macromolecule, and therefore characterizes the length of the molecular chain (molecule length) and predetermines its molecular weight.
The polymerization coefficient of cellulose of different origins is different. So, for wood cellulose it is 3000, for cotton - 12,000, for flax - 36,000 (approximately). This explains the great strength of cotton and flax fibers compared to wood cellulose fibers.
Alkaline cellulose is obtained by treating cellulose with a solution of sodium hydroxide. In this case, the hydrogen atoms of alcohol hydroxyls are partially or completely replaced by sodium atoms. Alkaline cellulose, without losing its fibrous structure, is characterized by increased chemical activity, which is used in the production of cellulose ethers, for example carboxymethylcellulose.
Carboxymethylcellulose (CMC) is an ether of cellulose and glycolic acid. The industrial method for the production of carboxymethylcellulose is based on the interaction of alkali cellulose with monochloroacetic acid.
Hemicelluloses are a cross between cellulose and starch. They are also polysaccharides. Hemicellulose molecules are built from monosaccharide residues: mannose (hexose) and xylose (pentose). Hemicelluloses do not have a fibrous structure. They serve as a reserve nutrient for plants and protect them from infections. Hemicelluloses swell in water and are relatively easily hydrolyzed even by very dilute acids; they dissolve in 18.5% alkali. Hemicelluloses are not harmful impurities of cellulose used for making paper. On the contrary, wood cellulose with a high content of hemicelluloses is easy to grind, and paper made from it has increased strength (especially surfaces), since hemicelluloses are a very good natural sizing agent.
Lignin is a chemically unstable substance: under the influence of light, moisture, oxygen, air and heat, lignin is destroyed, as a result of which plant fibers lose strength and darken. Lignin, unlike cellulose, dissolves in dilute acids and alkalis. Methods for producing cellulose from wood, straw, reed and other plant tissues are based on this property of lignin. The structure of lignin is very complex and has not yet been sufficiently studied; It is known that lignin is a natural polymer, the structural unit of which is the residue of a very reactive aromatic alcohol - β-oxyconiferyl.
It is difficult to imagine today's life without polymers - complex synthetic substances that have become widespread in various areas of human activity. Polymers are high-molecular compounds of natural or synthetic origin, consisting of monomers connected by chemical bonds. A monomer is a repeating unit of a chain that contains the parent molecule.
Organic high molecular weight compounds
Thanks to their unique properties, high-molecular compounds successfully replace natural materials such as wood, metal, stone in various spheres of life, conquering new areas of application. To systematize such a large group of substances, a classification of polymers according to various criteria has been adopted. These include composition, method of preparation, spatial configuration, and so on.
The classification of polymers according to their chemical composition divides them into three groups:
- Organic high molecular weight substances.
- Organoelement compounds.
- Inorganic high molecular weight compounds.
The largest group is represented by organic IUDs - resins, rubbers, vegetable oils, that is, products of animal as well as plant origin. The macromolecules of these substances in the main chain, along with carbon atoms, contain atoms of oxygen, nitrogen and other elements.
Their properties:
- have the ability to reverse deformation, that is, elasticity under low loads;
- at low concentrations they can form viscous solutions;
- change physical and mechanical characteristics under the influence of a minimum amount of reagent;
- under mechanical action, directional orientation of their macromolecules is possible.
Organoelement compounds
Organoelement IUDs, whose macromolecules include, in addition to atoms of inorganic elements - silicon, titanium, aluminum - and organic hydrocarbon radicals, are created artificially and do not exist in nature. The classification of polymers divides them, in turn, into three groups.
- The first group is substances in which the main chain is composed of atoms of some elements surrounded by organic radicals.
- The second group includes substances with a main chain containing alternating atoms of carbon and elements such as sulfur, nitrogen and others.
- The third group includes substances with organic main chains surrounded by various organoelement groups.
 An example is organosilicon compounds, in particular silicone, which has high wear resistance.
An example is organosilicon compounds, in particular silicone, which has high wear resistance.
Inorganic high-molecular compounds in the main chain contain oxides of silicon and metals - magnesium, aluminum or calcium. They do not have side organic atomic groups. The bonds in the main chains are covalent and ionic-covalent, which determines their high strength and heat resistance. These include asbestos, ceramics, silicate glass, quartz.
Carbon-chain and heterochain IUDs
Classification of polymers according to the chemical composition of the main polymer chain involves dividing these substances into two large groups.
- Carbon-chain, in which the main chain of the BMC macromolecule consists only of carbon atoms.
- Heterochain, in which in the main chain there are other atoms along with carbon atoms that give the substance additional properties.
Each of these large groups consists of the following subgroups, differing in the structure of the chain, the number of substituents, their composition, and the number of side branches:
- compounds with saturated bonds in chains, examples of which are polyethylene or polypropylene;
- polymers with unsaturated bonds in the main chain, for example polybutadiene;
- halogen-substituted high-molecular compounds – Teflon;
- polymer alcohols, an example of which is polyvinyl alcohol;
- IUDs obtained on the basis of alcohol derivatives, example - polyvinyl acetate;
- compounds derived from aldehydes and ketones, such as polyacrolein;

- polymers obtained from carboxylic acids, of which polyacrylic acid is a representative;
- substances derived from nitriles (PAN);
- high molecular weight substances derived from aromatic hydrocarbons, for example polystyrene.
Division according to the nature of the heteroatom
The classification of polymers may also depend on the nature of the heteroatoms; it includes several groups:
- with oxygen atoms in the main chain - polyesters and polyesters and peroxides;
- compounds containing nitrogen atoms in the main chain - polyamines and polyamides;
- substances with oxygen and also nitrogen atoms in the main chain, an example of which are polyurethanes;
- VMCs with sulfur atoms in the main chain - polythioethers and polytetrasulfides;
- compounds that have phosphorus atoms in the main chain.
Natural polymers
Currently, a classification of polymers by origin and chemical nature is also accepted, which divides them as follows:
- Natural, they are also called biopolymers.
- Artificial substances that are high molecular weight.
- Synthetic compounds.
Natural IUDs form the basis of life on Earth. The most important of them are proteins - the “building blocks” of living organisms, the monomers of which are amino acids. Proteins are involved in all biochemical reactions of the body; without them, the functioning of the immune system, blood clotting processes, the formation of bone and muscle tissue, energy conversion, and much more are impossible. Without nucleic acids, storage and transmission of hereditary information is impossible.

Polysaccharides are high molecular weight hydrocarbons that, together with proteins, participate in metabolism. Classification of polymers by origin allows us to classify natural high-molecular substances into a special group.
Artificial and synthetic polymers
Artificial polymers are obtained from natural ones through various methods of chemical modification to give them the necessary properties. An example is cellulose, from which many plastics are derived. The classification of polymers by origin characterizes them as artificial substances. Synthetic IUDs are produced chemically using polymerization or polycondensation reactions. Their properties, and therefore the scope of application, depend on the length of the macromolecule, that is, on the molecular weight. The larger it is, the stronger the resulting material. The classification of polymers by origin is very convenient. Examples confirm this.
Linear macromolecules
Any classification of polymers is quite arbitrary, and each has its own disadvantages, since it cannot reflect all the characteristics of a given group of substances. Nevertheless, it helps to somehow systematize them. The classification of polymers according to the shape of macromolecules presents them in the following three groups:
- linear;
- branched;
- spatial, which are also called mesh.
 The long, curved or spiral-shaped chains of linear IUDs give the substances some unique properties:
The long, curved or spiral-shaped chains of linear IUDs give the substances some unique properties:
- due to the appearance of intermolecular bonds, they form strong fibers;
- they are capable of large and long-term, but at the same time reversible deformations;
- an important property is their flexibility;
- when dissolved, these substances form solutions with high viscosity.
Branched macromolecules
Branched polymers also have a linear structure, but with many side branches, shorter than the main one. At the same time, their properties change:
- the solubility of substances with a branched structure is higher than that of linear ones; accordingly, they form solutions of lower viscosity;
- as the length of the side chains increases, the intermolecular forces become weaker, which leads to an increase in the softness and elasticity of the material;
- the higher the degree of branching, the more the physical properties of such a substance approach those of ordinary low-molecular compounds.
Three-dimensional macromolecules
Mesh high-molecular compounds are flat (staircase and parquet type) and three-dimensional. Flat materials include natural rubber and graphite. In spatial polymers there are cross-links - “bridges” between chains, forming one large three-dimensional macromolecule with extraordinary hardness.
An example would be diamond or keratin. Network high-molecular compounds are the basis of rubbers, some types of plastics, as well as adhesives and varnishes.
Thermoplastics and thermosets
The classification of polymers by origin and in relation to heating is intended to characterize the behavior of these substances when temperature changes. Depending on the processes occurring during heating, different results are obtained. If the intermolecular interaction weakens and the kinetic energy of the molecules increases, then the substance softens, turning into a viscous state. When the temperature decreases, it returns to its normal state - its chemical nature remains unchanged. Such substances are called thermoplastic polymers, for example polyethylene.
Another group of compounds is called thermosetting. The mechanism of the processes occurring in them when heated is completely different. If there are double bonds or functional groups, they interact with each other, changing the chemical nature of the substance. It cannot regain its original shape when cooled. An example is various resins.
Polymerization method
Another classification of polymers is based on the method of production. There are the following ways to receive an IUD:
- Polymerization, which can take place using an ionic reaction mechanism and a free radical one.
- Polycondensation.
Polymerization is the process of formation of macromolecules by sequential connection of monomer units. They are usually low molecular weight substances with multiple bonds and cyclic groups. During the reaction, the double bond or bond in the cyclic group is broken, and new ones are formed between these monomers. If a reaction involves monomers of the same type, it is called homopolymerization. When different types of monomers are used, a copolymerization reaction occurs.

The polymerization reaction is a chain reaction that can occur spontaneously, but active substances are used to accelerate it. With the free radical mechanism, the process occurs in several stages:
- Initiation. At this stage, through light, heat, chemical or some other influence, active groups - radicals - are formed in the system.
- Increase in chain length. This stage is characterized by the addition of further monomers to radicals to form new radicals.
- Chain termination occurs when active groups interact to form inactive macromolecules.
It is impossible to control the moment of chain termination, and therefore the resulting macromolecules have different molecular weights.
The principle of operation of the ionic mechanism of the polymerization reaction is the same as the free radical one. But here cations and anions act as active centers, so a distinction is made between cationic and anionic polymerization. In industry, the most important polymers are produced by radical polymerization: polyethylene, polystyrene and many others. Ionic polymerization is used in the production of synthetic rubbers.
Polycondensation
The process of formation of a high-molecular compound with the separation of some low-molecular substances as a by-product is polycondensation, which differs from polymerization in that the elemental composition of the resulting macromolecule does not correspond to the composition of the initial substances participating in the reaction. They can only involve compounds with functional groups, which, when interacting, split off a molecule of a simple substance and form a new bond. Polycondensation of bifunctional compounds produces linear polymers. When polyfunctional compounds participate in the reaction, BMCs with a branched or even spatial structure are formed. Low molecular weight substances formed during the reaction also interact with intermediate products, causing chain termination. Therefore, it is better to remove them from the reaction zone. 
Certain polymers cannot be obtained by known polymerization or polycondensation methods, since there are no required starting monomers capable of participating in them. In this case, the synthesis of the polymer is carried out with the participation of high-molecular compounds containing functional groups that are able to react with each other.
Every day the classification of polymers becomes more complicated, as more and more new types of these amazing substances with predetermined properties appear, and people can no longer imagine their life without them. However, another problem arises, no less important - the possibility of their easy and cheap disposal. Solving this problem is very important for the existence of the planet.