What is an electric current conductor
The term has two meanings: 1) an electrically conductive substance (for example, metal or electrolyte), 2) a part, product or structure that allows the transmission of electricity.
The first value is used in physics and materials science, where all materials are divided into conductors, dielectrics and semiconductors by their electrical conductivity. In power engineering, they often use the second meaning of this term. The transmission of electrical energy through conductors can occur - from one element of a source, converter or receiver of electrical energy to another through connecting conductors over a distance of several nanometers (for example, in integrated circuits) to several meters (for example, in powerful power equipment); - from one element of an electrical installation to another or from one electrical installation to another along electric lines at a distance of several meters (for example, within the same installation) to several thousand kilometers (between large power systems).
The set of lines and their nodes in an electrical installation is called electrical wiring, and the set of lines and their nodes, interconnecting electrical installations, - electric network. By purpose and length in power systems, system-forming (main) and distribution networks are distinguished, at enterprises there are inter-workshop and workshop networks, etc.
The transmission of an electric charge through a conductor (linen thread) was discovered in 1663 by the mayor of Magdeburg, Otto von Guericke, 1602–1686, who had made the first electrostatic generator in the world that same year. A more detailed study of electrical phenomena began in the 18th century, and on July 2, 1729, English amateur physicist Stephen Gray (1666–1735) paved using hemp rope 80.5 feet long on horizontal silk to test the transmission of electricity. cords (Fig. 4.5.1); he created the world's first electric line. On July 14, he held a public demonstration of the line, which was already 650 feet long, with hemp rope still running along silk cords stretched between the pillars (first overhead line). The experience, despite the very poor conductivity of the wire, surprisingly failed; the rope was obviously (thanks to the English climate) quite wet. Gray also first introduced the classification of substances as conducting and non-conducting. After 10 years (in 1739), another English physicist, Jean Theophile Desaguliers (1683–1744), introduced the concept of conductor. The first overhead line with metal (iron) wires was built in 1744 in Erfurt (Erfurt, Germany) by the German professor of philosophy Andreas Gordon (1712–1751), and the first experimental cable (telegraph) line was laid in 1841 in St. Petersburg Boris Semenovich Jacobi (Moritz Hermann Jacobi).
Fig. 1. The principle of the device of the first electric line of Stephen Gray. 1 hemp rope (wire), 2 silk cords (insulators)
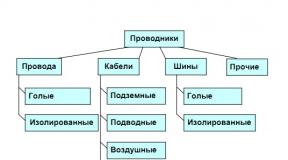
Both flexible and rigid conductors are used in power transmission technology. The first include various wires and cablesto the second tires. Wires and busbars may be insulated or uninsulated (bare). Insulated wires and cables may contain from one to several current-carrying veinsisolated from each other.
Hallmark cable is a sealed sheath made of polymeric materials (for example, polyvinyl chloride) or metal (currently most often aluminum, formerly mainly lead), which protects the cores from the harmful effects of the environment. A simplified classification of conductors according to their flexibility, insulation and scope is shown in Fig. 2.
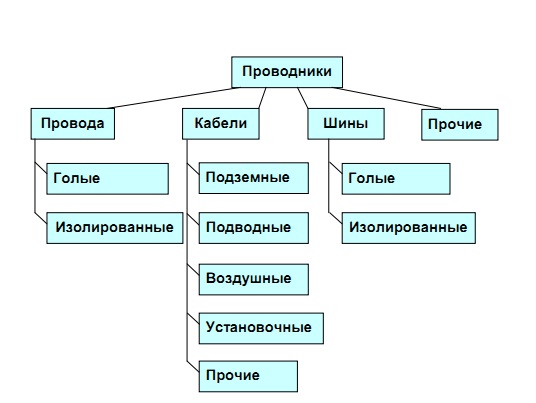
Fig. 2. Classification of conductors (simplified)
The metal part of the cores, depending on the cross-section and the required flexibility, can be massive or consist of wires; the diameter of the wires can vary from tenths of a millimeter (in thin-wire cores) to several millimeters. Conductors required
High conductivity
- good contact properties,
- high dielectric strength,
- sufficient mechanical strength,
- sufficient flexibility (in the case of wires and cables),
- long-term chemical stability,
- sufficient resistance to heat,
- sufficient heat capacity,
- protection from external influences,
- harmless to the environment,
- ease of use in electrical work,
- moderate cost.
Of conductive materials, these requirements are best met
- pure (without any impurities) copper,
- pure aluminum (for reasons of reliability, starting from 16 mm2),
- in the wires of overhead lines
- combinations of aluminum and steel.
Of the insulation materials most commonly used
- polyethylene n,
- polyvinyl chloride n, which resists ignition better than other materials, but which contains toxic and environmentally hazardous chlorine, - synthetic (including especially heat-resistant organosilicon) rubbers.
Conductors (and conductors of stranded conductors) are divided according to their purpose
- on the working conductors (to which in the case alternating current phase and neutral conductors; some networks or installations may not have neutral conductors);
- on the protective conductorsnecessary to ensure the safety of people;
- on the auxiliary conductors (e.g. for control, communication, or signaling). The working conductors can all be isolated from the ground, but often one of them (usually neutral) is grounded. Such working grounding achieves a lower and evenly distributed voltage of the phase conductors relative to the ground, which, for example, in high voltage networks can reduce the cost of insulation.
Protective conductors are provided for reliable grounding of those parts of electrical installations that, if insulation is broken, may be energized (open conductive parts). Such protective grounding should eliminate the occurrence of hazardous voltage between these parts and the ground and thereby exclude the possibility of electric shock to people. In low voltage electrical networks, the combination of protective and neutral conductors was previously practiced; these conductors are currently separated for reliability and safety reasons.
All objects around us are made up of extremely small particles of matter that are invisible to the eye — molecules in which even smaller particles — atoms — are combined. An atom in turn consists of an electrically charged nucleus and electrons. The structure of the atom is quite complex: in its center is a positively charged nucleus, around which negatively charged electrons move.
In the absence of external influence on the atoms, the substance that they form is electrically neutral: the positive charge of the nucleus of each atom is balanced by the negative charges of its electrons. But if a lack of electrons is artificially created in an atom of a substance, then such a substance will have a positive charge, and, conversely, if an excess of electrons is created, such a substance will become negatively charged.
If the electrical neutrality of an atom is violated, its state is extremely unstable, then the atom tends to either give away excess electrons to other atoms, or, conversely, attach the missing electrons to itself. Therefore, if we connect with a conductor substances with different charges, the conductor begins to move electrons from a negatively charged substance to a positively charged substance, - electricity. This movement of electrons, or, in other words, the electric current in the conductor, will be until the charges of the substances connected by the conductor balance each other. In order to continuously maintain an electric current, it is necessary to continuously maintain an excess of electrons at one end of the conductor, and a lack of them at the other.
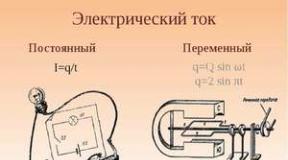
Electricity is a type of energy along with chemical, thermal, mechanical, etc. Electric energy is subject to the general law of conservation and conversion of energy. Electric energy can be converted into chemical, mechanical and other types of energy, which in turn can also be converted into electrical energy.
Consider, for example, how chemical energy is converted into electrical energy.
If a solution of sulfuric acid and water is poured into a glass vessel 2 and the copper and zinc plates (electrodes) are lowered into it, then we obtain the simplest galvanic cell.
Fig. Galvanic cell: 1 - an electric bulb; 2 - vessel
When the ends (poles) of the copper and zinc plates are closed, an electric current flows through the circuit. The current effect can be seen if a light bulb 1 is connected to the plates: the thread of the bulb heats up and glows.
The current appeared because a chemical interaction began between the electrodes and the acid solution. As a result of this interaction, an excess of electrons is formed on the zinc electrode, and a lack of electrons on the copper electrode.
The electric current in this case moves both through the wires of the light bulb (external circuit) and inside the element through a solution of sulfuric acid (internal circuit) - from a negatively charged zinc plate to a copper positively charged plate.
In practice, according to tradition, the technical direction electric current it is conventionally considered to be the opposite - from the positive pole to the negative.
An electric current moves under the influence of an electromotive force. This force is spent on overcoming resistance to the movement of electrons both in the external circuit and in the internal.
The part of the electromotive force that goes to overcome the resistance of the external circuit, causing the movement of current in the circuit, is called voltage.
Electromotive force and voltage are expressed in volts (c) and are measured by special devices - voltmeterami.
The amount of electricity flowing through the conductor per unit of time (per second) determines the amount of current. It is expressed in amperes (a) and is measured by a special device - ammeterohm
The resistance of the conductor to the movement of electric current is called electrical resistance. Resistance expressed in ohms (ohms) and measured with an ohmmeter.
Various substances exhibit unequal resistance to the passage of electric current. So, for example, copper and aluminum conduct electricity well; glass, plastics, porcelain practically do not conduct it. By the ability to conduct electric current, all substances are usually divided into conductors (metals, coal, solutions of acids, alkalis, etc.) and non-conductors (rubber, glass, ebonite, etc.).
In a closed electrical circuit voltage, its value and circuit resistance are interconnected by a certain ratio (Ohm's law); the higher the voltage of the current source and the lower the resistance of the conductor, the greater the magnitude of the electric current.
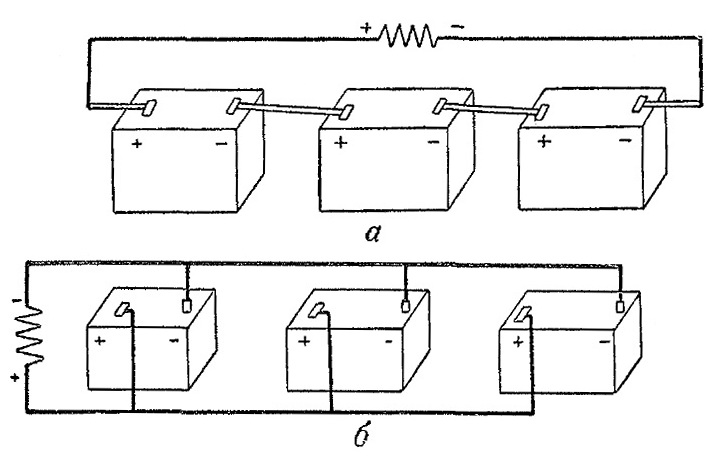
Fig. Battery connection diagram: a - serial connection; b - parallel connection
This ratio can be arbitrarily compared with the movement of water flowing through pipes from a water tower. The higher the water tower, which creates the pressure (voltage) of the water, and the larger the size of the pipes through which water is supplied (i.e., its resistance to movement is small), the more water flows per unit time.
In the vehicle’s electrical system, a series, parallel or mixed connection of sources and consumers of electric current is used to change the magnitude and voltage of the current and the resistance to its movement.
Consider the features of serial and parallel connections on the example of two identical sources of electric current with a voltage of 2 V.
If the current sources are interconnected in series (Fig. A), i.e., the minus terminal of the first source is connected to the plus terminal of the second, the minus terminal of the second and the plus terminal of the third, and the plus terminal of the first source is connected through any consumer to the negative terminal of the third , then the total voltage of current sources will be 6 V.
If the current sources are interconnected in parallel (Fig. B), i.e. If you connect the positive terminals of the sources into one node and connect the negative terminals into one node, and connect the ends of the wires from the nodes to the current consumer, the total voltage of the current sources will not increase, it will be 2 V. But in the latter case, they will be able to transfer three times more current to the external circuit than in the first case, when the current sources were connected in series.
Electric consumers can also be connected in series or in parallel. With a series connection of current consumers, their total resistance to the movement of current increases, while in parallel it decreases.
This phenomenon can again be compared with the movement of water through several pipes having the same internal diameters and lengths.
If water flows through pipes of the same diameter, arranged one after another in series, its resistance to movement is great; if water flows simultaneously through all pipes in parallel, its resistance to movement is much less.
The amount of electricity passing through any current consumer is determined by the product of the current value (in amperes) by the duration of the current (in hours) and is expressed in ampere-hours.
Moving along the conductor, the current does the work, for example, heats the conductor, spending electrical energy for this. The work performed by the current depends on the voltage, current magnitude and duration. The work of the electric current is determined by the product of the voltage (in volts) by the amount of current (in amperes) and the duration of the current (in hours) and is expressed in watt hours.
Electric current called the work done by him in 1 sec. It is the product of voltage (in volts) and current (in amperes) and is expressed in watts. The power of electric current can also be expressed in horsepower: 1 horsepower is 736 watts.
Each person, constantly using electrical appliances, is faced with the properties of electrical conductivity, namely:
All substances depending on electrical conductivity are divided into conductors, semiconductors and dielectrics:
1. conductors - which pass electric current;
2. dielectrics - possess insulating properties;
3. semiconductors - combine the characteristics of the first two types of substances and change them depending on the applied control signal.
TO conductors include those substances that have in their structure a large number of free, rather than connected, electric charges capable of starting movement under the influence of an external force. They can be in solid, liquid or gaseous state. The most excellent conductors of electric current are metals.R solutions of salts and acids, moist soil, bodies of people and animals are also good conductors of electric charges.
If we take two conductors between which a potential difference is formed and connect a metal wire inside them, then an electric current will flow through it. Its carriers will be free electrons that are not held by the bonds of atoms. They characterize the magnitude of the electrical conductivity or the ability of any substance to pass through itself electric charges - current.
The value of electrical conductivity is inversely proportional to the resistance of the substance and is measured by the corresponding unit: siemens (cm).
1 cm \u003d 1/1 ohm.
In nature, charge carriers can be:
electrons;
ions;
holes.
According to this principle, electrical conductivity is divided into:
electronic;
ionic;
hole.
The quality of the conductor makes it possible to evaluate the dependence of the current flowing in it on the value of the applied voltage. It is commonly called by the designation of units of measurement of these electrical quantities - current-voltage characteristic.
Conductors with electronic conductivity (conductors of the 1st kind)
The most common representative of this type are metals. They create an electric current solely due to the movement of the electron flow.
When electric current passes through metal conductors, neither their mass nor their chemical composition changes. Consequently, metal atoms do not participate in the transfer of electric charges. Studies of the nature of electric current in metals have shown that the transfer of electric charges in them is carried out only by electrons.
Inside metals, they are in two states:
bonded by atomic cohesion forces;
free.
Electrons held in orbit by the attractive forces of an atomic nucleus, as a rule, do not participate in the creation of an electric current under the action of external electromotive forces. Otherwise, free particles behave.
If EMF is not applied to the metal conductor, then free electrons move randomly, randomly, in any directions. Their movement is due to thermal energy. It is characterized by different speeds and directions of movement of each particle at any time.
When an external field energy with intensity E is applied to the conductor, then a force directed opposite to the acting field acts on all the electrons together and each separately. It creates a strictly oriented movement of electrons, or in other words - an electric current.
The current-voltage characteristic of metals is a straight line that fits into the Ohm's law for the site and the complete circuit.
In addition to pure metals, other substances also possess electronic conductivity. These include:
alloys;
individual carbon modifications (graphite, coal).
All of the above substances, including metals, are classified as 1st kind conductors. Their electrical conductivity is in no way connected with the transfer of mass of matter due to the passage of electric current, but is caused only by the movement of electrons.
If metals and alloys are placed in an environment of ultra-low temperatures, then they become superconducting.
Conductors with ionic conductivity (conductors of the 2nd kind)
This class includes substances in which an electric current is created due to the movement of charges by ions. They are classified as conductors of the second kind.
solutions of alkalis, salts of acids;
melts of various ionic compounds;
various gases and vapors.
Electric current in a liquid
Conducting electric currents in liquid media in which electrolysis occurs - the transfer of a substance together with charges and its deposition on electrodes is commonly called electrolytes, and the process itself is called electrolysis.
It occurs under the influence of an external energy field due to the application of a positive potential to the anode electrode and a negative potential to the cathode.
Ions inside liquids are formed due to the phenomenon of electrolytic dissociation, which consists in the splitting of part of the molecules of a substance with neutral properties.
Under the action of an applied voltage to the electrolyte, cations begin to move strictly toward the cathode, and anions - toward the anode. In this way, chemically pure, without impurities, copper is obtained, which is released at the cathode.
In addition to liquids, solid electrolytes exist in nature. They are called superionic conductors (superionics) having a crystalline structure and the ionic nature of chemical bonds, which causes high electrical conductivity due to the movement of ions of the same type.
Hole Conductors
These include:
germanium;
selenium;
silicon;
compounds of individual metals with tellurium, sulfur, selenium and some organic substances.
They got the name semiconductors and belong to group No. 1, that is, they do not form a transfer of matter during the flow of charges. To increase the concentration of free electrons inside them, it is necessary to spend additional energy on the separation of bound electrons. It is called ionization energy.
The semiconductor has an electron-hole transition. Due to it, the semiconductor passes current in one direction and blocks in the opposite direction when an opposite external field is applied to it.
Semiconductor structure
Conductivity in semiconductors is:
1. own;
2. impurity.
The first type is inherent in structures in which charge carriers appear in the process of ionization of atoms of their substance: holes and electrons. Their concentration is mutually balanced.