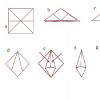
Nomenclature of peptides and polypeptides. Natural peptides: glutathione, carnosine, anserine, gramicidin s, oxytocin, enkephalins. Siberian State Medical University Gli Ile Arg Met Tre
"Metabolism and energy of the cell" - Definition. Plastic exchange. The digestive organs. Preparing students for open-ended assignments. Chemical transformations. Tasks with the answer "yes" or "no". Metabolism. Metabolism. Misspelled text. A task with a detailed answer. Test tasks. Energy exchange.
"Metabolism" - Properties of the genetic code. Molecular weight of one amino acid. Genetic code. The initial part of the molecule. Plastic exchange. Transcription. Protein. DNA. Determine the length of the corresponding gene. Assimilation and dissimilation reactions. A section of the right DNA strand. Give definitions of terms. Autotrophs. Protein biosynthesis. What is the primary structure of the protein? A protein composed of 500 monomers. Broadcast.
"Energy metabolism" Grade 9 "- Glucose is the central molecule of cellular respiration. ATP in numbers. The concept of energy metabolism. Autotrophs. PVC - pyruvic acid С3Н4О3. ATP structure. ATP is a universal source of energy in the cell. Conversion of ATP to ADP. Fermentation is anaerobic breathing. Metabolism. Energy metabolism in the cell. Fermentation. Energy exchange (dissimilation). Mitochondria. The aerobic stage is oxygen. The summary equation of the aerobic stage.
“Stages of energy metabolism” - The summary equation. Types of organisms nutrition. Cleavage process. Metabolism. Oxidation of PVC. Electron transport chain. Release of energy. Krebs cycle. Describe the reactions. Oxidative decarboxylation. Catabolism. Aerobic breathing. Stages of aerobic respiration. Preparatory stage. Oxygen breakdown. Solar energy. Where does ATP synthesis take place? Oxygen-free stage. Fill in the gaps in the text.
"Metabolism of carbohydrates" - The result of the Krebs cycle. Triose phosphate isomerase. Sucrose. Chemiosmotic model of ATP synthesis. Factors affecting enzyme activity. Metabolism. Glycolysis. Aldolase. Enzyme classification. Stocking up. Stages of glucose oxidation. Forking. Enzymes. Protein components of the mitochindrial ETC. Enzymes. The main stages of carbohydrate metabolism. Enolaz. Glycogen synthesis. Oxidation of acetyl-CoA to CO2.
"Energy metabolism" - The process of energy metabolism. Glycolysis. Energy released in glycolysis reactions. Enzymes of the oxygen-free stage of energy exchange. The fate of the PVC. Lactic acid fermentation. Lactic acid. Biological oxidation and combustion. Oxidation of substance A. Preparatory stage. Reiteration. Combustion. Energy exchange.
Protein - high molecular weight natural polymers, consisting of amino acid residues linked by a peptide bond; are the main constituent part of living organisms and the molecular basis of vital processes.
More than 300 different amino acids are known in nature, but only 20 of them are part of the proteins of humans, animals and other higher organisms. Each amino acid has carboxyl group, amino group in the α-position (at the 2nd carbon atom) and radical (side chain), different for different amino acids. At physiological pH (~ 7.4), the carboxyl group of amino acids usually dissociates, and the amino group is protonated.
All amino acids (with the exception of glycine) contain an asymmetric carbon atom (that is, such an atom, all four valence bonds of which are occupied by different substituents, it is called a chiral center), therefore, they can exist in the form of L- and D-stereoisomers (the reference is glyceraldehyde ):

Only L-amino acids are used for the synthesis of human proteins. In proteins with a long lifetime, the L-isomers can slowly acquire the D-configuration, and this occurs at a certain rate characteristic of each amino acid. Thus, dentin proteins of teeth contain L-aspartate, which transforms into D-form at a human body temperature at a rate of 0.01% per year. Since the dentin of teeth is practically not exchanged and is not synthesized in adults in the absence of trauma, the age of a person can be determined by the content of D-aspartate, which is used in clinical and forensic practice.
All 20 amino acids in the human body differ in structure, size and physical and chemical properties radicals attached to the α-carbon atom.
The structural formulas of 20 proteinogenic amino acids are usually given in the form of the so-called proteinogenic amino acid tables:

IN recent times for the designation of amino acids, one-letter designations are used; to memorize them, a mnemonic rule is used (fourth column).
Chapter III. PROTEINS
§ 6. AMINO ACIDS AS STRUCTURAL ELEMENTS OF PROTEINS
Natural amino acids
Amino acids in living organisms are found mainly in proteins. Proteins are built mainly by twenty standard amino acids. They are a-amino acids and differ from each other in the structure of side groups (radicals), denoted by the letter R:
The variety of amino acid side radicals plays a key role in the formation of the spatial structure of proteins, during the functioning of the active center of enzymes.
The structure of standard amino acids is shown at the end of the paragraph in Table 3. Natural amino acids have trivial names, which are inconvenient to operate with when recording the structure of proteins. Therefore, three-letter and one-letter designations have been introduced for them, which are also presented in Table 3.
Spatial isomerism
All amino acids, with the exception of glycine, have a chiral a-carbon atom, i.e. they are characterized by optical isomerism. Table The 3 chiral carbon atom is indicated by an asterisk. For example, for alanine, the Fischer projections of both isomers are as follows:
For their designation, as well as for carbohydrates, the D, L-nomenclature is used. Proteins contain only L-amino acids.
L- and D-isomers can mutually transform into each other. This process is called racemization.
Interesting to know! In the protein of teeth - dentin -L-aspartic the acid spontaneously racemizes at human body temperature at a rate of 0.10% per year. During the formation of teeth, dentin contains onlyL-aspartic acid, in an adult, as a result of racemization,D-aspartic acid. The older the person, the higher the D-isomer content. Having determined the ratio of D- and L-isomers, it is possible to accurately determine the age. Thus, the inhabitants of the mountain villages of Ecuador were exposed, who ascribed to themselves too great an age.
Chemical properties
Amino acids contain amino and carboxyl groups. Because of this, they exhibit amphoteric properties, that is, properties of both acids and bases.
When an amino acid is dissolved in water, such as glycine, its carboxyl group dissociates to form a hydrogen ion. Further, the hydrogen ion is attached due to the lone pair of electrons at the nitrogen atom to the amino group. An ion is formed, in which positive and negative charges are simultaneously present, the so-called zwitterion:
This form of the amino acid is predominant in a neutral solution. In an acidic environment, an amino acid, adding a hydrogen ion, forms a cation:
In an alkaline environment, an anion is formed:
Thus, depending on the pH of the medium, an amino acid can be positively charged, negatively charged, and electrically neutral (if the positive and negative charges are equal). The pH value of a solution at which the total charge of an amino acid is zero is called isoelectric point of this amino acid. For many amino acids, the isoelectric point lies near pH 6. For example, the isoelectric points of glycine and alanine are 5.97 and 6.02, respectively.
Two amino acids can react with each other, resulting in the cleavage of a water molecule and a product called dipeptide:
The bond connecting two amino acids is called peptide bond... Using the letter designations of amino acids, the formation of a dipeptide can be schematically represented as follows:
Similarly formed tripeptides, tetrapeptides etc.:
H 2 N - lys - ala - gly - COOH - tripeptide
H 2 N - trp - his - ala - ala - COOH - tetrapeptide
H 2 N - tyr - lys - gly - ala - leu - gly - tp - COOH - heptapeptide
Peptides consisting of a small number of amino acid residues have a common name oligopeptides.
Interesting to know! Many oligopeptides have high biological activity. These include a number of hormones, for example, oxytocin (nanopeptide) stimulates uterine contraction, bradykinin (nanopeptide) suppresses inflammatory processes in tissues. The antibiotic gramicidin C (cyclic decapeptide) disrupts the regulation of ionic permeability in the membranes of bacteria and thereby kills them. Fungal poisons amanitins (octapeptides), blocking protein synthesis, can cause severe poisoning in humans. Aspartame, the methyl ester of aspartylphenylalanine, is widely known. Aspartame has a sweet taste and is used to impart sweet taste to various foods and drinks.
Amino acid classification
There are several approaches to the classification of amino acids, but the most preferable is the classification based on the structure of their radicals. There are four classes of amino acids containing the following types of radicals; one) non-polar (or hydrophobic); 2) polar uncharged; 3) negatively charged and 4) positively charged:
Non-polar (hydrophobic) amino acids include non-polar aliphatic (alanine, valine, leucine, isoleucine) or aromatic (phenylalanine and tryptophan) R-groups and one sulfur-containing amino acid, methionine.
Polar uncharged amino acids, in comparison with non-polar ones, dissolve better in water, are more hydrophilic, since their functional groups form hydrogen bonds with water molecules. These include amino acids containing a polar HO group (serine, threonine and tyrosine), an HS group (cysteine), an amide group (glutamine, asparagine), and glycine (the R group of glycine, represented by one hydrogen atom, is too small to compensate strong polarity of the a-amino group and a-carboxyl group).
Aspartic and glutamic acids are negatively charged amino acids. They contain two carboxyl and one amino group, therefore, in the ionized state, their molecules will have a total negative charge:
Lysine, histidine and arginine belong to positively charged amino acids; in ionized form they have a total positive charge:
Depending on the nature of the radicals, natural amino acids are also subdivided into neutral, sourand the main... Neutral and non-polar are non-charged, acidic - negatively charged, basic - positively charged.
Ten of the 20 amino acids that make up proteins can be synthesized in the human body. The rest should be contained in our food. These include arginine, valine, isoleucine, leucine, lysine, methionine, threonine, tryptophan, phenylalanine, and histidine. These amino acids are called irreplaceable. Essential amino acids are often included in food supplements and used as medicines.
Interesting to know! An extremely important role is played by the balance of human nutrition in terms of amino acids. With a lack of essential amino acids in food, the body self-destructs. In this case, primarily the brain suffers, which leads to various diseases of the central nervous system, mental disorders. A young growing organism is especially vulnerable. So, for example, when the synthesis of tyrosine from phenylalanine is disturbed, children develop a severe disease of finylpyruvic oligophrenia, which causes severe mental retardation or the death of a child.
Table 3
Standard amino acids
|
Amino acid (trivial name) |
Legend |
Structural formula |
||
|
Latin |
||||
|
three-letter |
one-letter-vein |
|||
|
NON-POLAR (HYDROPHOBIC) |
||||
|
Isoleucine |
|
|||
|
Phenylalanine |
||||
|
Tryptophan |
|
|||
|
Methionine |
||||
|
POLAR UNCHARGED |
||||
|
Asparagine |
|
|||
|
Glutamine | ||||
differs from a similar polypeptide in TSH in cattle
amino acid residues and the absence of the C-terminal methionine. By-
the properties of the hormone are explained by the presence of the β-subunit of TSH in the complex
with an α-subunit. It is assumed that the action of thyrotropin is carried out
it is, like the action of other hormones of a protein nature, through
binding to specific receptors of plasma membranes and ac-
tivation of the adenylate cyclase system (see below).
Gonadotropic hormones (gonadotropins)
Gonadotropins include follicle-stimulating hormone (FSH,
follitropin) and luteinizing hormone (LH, lutropin), or hormone,
stimulating interstitial cells *. Both hormones are synthesized
in the anterior lobe of the pituitary gland and are, like thyrotropin, complex
proteins - glycoproteins with a pier. weighing 25000. They regulate the ste-
rhoid and gametogenesis in the gonads. Follitropin induces maturation
follicle growth in the ovaries in females and spermatogenesis in males. Lutropin
in females, stimulates the secretion of estrogen and progesterone, as well as rupture
follicles with the formation of a yellow body, and in males - the secretion of dough
sterone and the development of interstitial tissue. Biosynthesis of gonadotropins,
as noted, it is regulated by the hypothalamic hormone gonadolibe
The chemical structure of the lutropin molecule has been fully deciphered.
Lutropin consists of two α- and β-subunits. Α-subunit structure
hormone in most animals is the same. So, in a sheep, it contains 96
amino acid residues and 2 carbohydrate radicals. In humans, α-subdivisions
the hormone is shortened by 7 amino acid residues from the N-terminus and differs
22 amino acids are naturally occurring. The sequence
amino acids in the β-subunits of pig and human lutropin. α- and β-Subb-
units are individually devoid of biological activity (by analogy
with most of the enzyme subunits). Only their complex, education
which, most likely, is predetermined by their primary structure,
leads to the formation of a biologically active macromolecular structure
tours due to hydrophobic interactions.
Lipotropic hormones (LTH, lipotropins)
Among the hormones of the anterior pituitary gland, the structure and function of which
found out in the last decade, lipotropins should be noted, in particular
β- and γ-LTG. The primary structure of β-lipo
the path of the sheep and the pig, the molecules of which consist of 91 amino acids
remainder and have significant species differences in the sequence
amino acids. The biological properties of β-lipotropin include fat
mobilizing effect, corticotropic, melanocyte-stimulating and hy-
pocalcemic activity and, in addition, an insulin-like effect,
expressed in an increase in the rate of glucose utilization in tissues.
It is assumed that the lipotropic effect is carried out through the system
* The group of gonadotropins also includes chorionichesky gonadotropin human
century (hCG), synthesized by the cells of the placenta and represented by glycoprotein.
adenylate cyclase-cAMP-protein kinase, the final stage of action
which is the phosphorylation of inactive triacylglycerol lipase.
This enzyme, after activation, breaks down neutral fats into
diacylglycerol and higher fatty acid (see chapter 11).
The listed biological properties are not due to β-lipotropy
mr., who turned out to be deprived of hormonal activity, and the products of his
decay formed by limited proteolysis. It turned out that
in the brain tissue and in the intermediate lobe of the pituitary gland, biological
internally active peptides endowed with an opiate-like effect. Privo-
dim the structures of some of them:
H–Shooting gallery–Gley–Gley–Hair dryer–Met – OH
Methionine-enkephalin
H–Shooting gallery–Gley–Gley–Feng – Lei – OH
Leucine-enkephalin
H–Shooting gallery–Gley–Gley–Hair dryer–Met – Tre – Ser – Glu – Liz – Ser – Gln – Tre – Pro–
Lei – Val – Tre – Lei – Fen – Liz – Asn – Ala – Ile – Val – Liz – Asn – Ala – Gis–
Liz-Liz-Gly-Gln-OH
β-Endorphin
The common type of structure for all three compounds is tetra-
peptide sequence at the N-terminus. It has been proved that β-endorphin (31
AMK) is formed by proteolysis from a larger pituitary
the hormone β-lipotropin (91 AMK); the latter, together with ACTH, is formed from
common precursor - a prohormone, called pro about p and about k ortin about m
(is thus a preprohormone) having a molecular
mass 29 kDa and numbering 134 amino acid residues. Biosynthesis
and the release of proopiocortin in the anterior pituitary gland is regulated
corticoliberin of the hypothalamus. In turn, from ACTH and β-lipo
pathway through further processing, in particular limited pro
theolysis, respectively α- and β-melanocyte-stimulating hormone
mones (α- and β-MSH). Using DNA cloning techniques as well
method for determining the primary structure of Sanger nucleic acids
a number of laboratories have disclosed the nucleotide sequence
mRNA-precursor of proopiocortin. These studies can serve
live the basis for the targeted production of new biologically active
hormonal medicinal products.
Below are the peptide hormones formed from β-lipotro-
pin by specific proteolysis.
Plot β -lipotropin
Peptide hormone
γ-Lipotropin
Met-enkephalin
α-Endorphin
γ-Endorphin
δ-Endorphin
β-Endorphin
Considering the exceptional role of β-lipotropin as a precursor
of the listed hormones, we give the primary structure of β-lipotropin
pigs (91 amino acid residues):
N-Glu-Lei-Ala-Gli-Ala-Pro-Pro-Glu-Pro-Ala-Arg-Asp-Pro-Glu–
Ala-Pro-Ala-Glu-Gli-Ala-Ala-Ala-Arg-Ala-Glu-Lei-Glu-Tir–
Gli-Lei-Val-Ala-Glu-Ala-Glu-Ala-Ala-Glu-Liz-Liz-Asp-Glu–
Gly – Pro – Tyr – Liz – Met – Glu – Gis – Phen – Arg – Trp – Gly – Ser – Pro – Pro–
Liz – Asp – Liz – Arg – Tyr – Gly – Gly – Fen – Met – Tre – Ser – Glu – Liz – Ser–
Gln – Tre – Pro – Lei – Val – Tre – Lei – Fen – Liz – Asn – Ala – Ile – Val – Liz–
Asn – Ala – Gis – Liz – Liz – Gly – Gln – OH
Increased interest in these peptides, in particular enkephalins
and endorphins, dictated by their extraordinary ability, like morphine,
relieve pain. This area of \u200b\u200bresearch is the search for new
native peptide hormones and (or) their targeted biosynthesis - is
interesting and promising for the development of physiology, neurobiology,
neurology and clinics.
Parathyroid hormones
(PARATHORMONES)
The hormones of a protein nature also include parathyroid hormone
(parathyroid hormone), more precisely, a group of parathyroid hormones, differing in sequence
amino acidity. They are synthesized by the parathyroid glands -
mi. Back in 1909, it was shown that the removal of the parathyroid glands
causes tetanic convulsions in animals against the background of a sharp fall
plasma calcium concentration; the introduction of calcium salts prevented
the death of animals fell. However, only in 1925 from the parathyroid glands
an active extract was isolated, causing a hormonal effect -
in 1970 from the parathyroid glands of cattle; then was
its primary structure is determined. It was found that parathyroid hormone is synthesized
in the form of a precursor (115 amino acid residues).
hormone, however, the primary product of the gene turned out to be
25 amino acid residues. Bovine parathyroid hormone molecule contains 84
amino acid residue and consists of one polypeptide chain.
It was found that parathyroid hormone is involved in the regulation of the concentration of cation
new calcium and associated anions of phosphoric acid in the blood. how
it is known that the concentration of calcium in the blood serum is a chemical
constants, its daily fluctuations do not exceed 3–5% (normally 2.2–
2.6 mmol / L). The biologically active form is considered ionized
calcium, its concentration ranges from 1.1-1.3 mmol / l. Jonah
calcium turned out to be essential factors that are not replaceable by other
cations for a number of vital physiological processes:
contraction, neuromuscular excitement, blood coagulation, penetration
the value of cell membranes, the activity of a number of enzymes, etc. therefore
any changes in these processes due to long-term
a lump of calcium in food or a violation of its absorption in the intestine, lead
to enhance the synthesis of parathyroid hormone, which promotes leaching
calcium salts (in the form of citrates and phosphates) from bone tissue and the corresponding
venno to the destruction of mineral and organic components of bones.
Another target organ for parathyroid hormone is the kidney. Parathyroid hormone reduces
reabsorption of phosphate in the distal tubules of the kidney and increases the tubule
reabsorption of calcium.
It should be noted that in the regulation of Ca concentration
in extracellular
fluids play a major role in three hormones: parathyroid hormone, calcitonin,
] - derivative D
(see chapter 7). All three hormones regulate the level
But the mechanisms of their action are different. So, the main role of calcitri-
la is to stimulate the absorption of Ca
and phosphate in the intestines,
moreover, against the concentration gradient, while parathyroid hormone
promotes their release from bone tissue into the blood, calcium absorption
in the kidneys and excretion of phosphate in the urine. The role of calcitonin has been less studied
in regulation of Ca homeostasis
in the body. It should also be noted that
calcitriol on the mechanism of action at the cellular level is similar to
the action of steroid hormones (see below).
It is considered proven that the physiological effect of parathyroid hormone on
kidney cells and bone tissue is realized through the adenylate cyclase system
THYROID HORMONES
The thyroid gland plays an extremely important role in metabolism.
This is evidenced by a sharp change in basal metabolism, observed
mine for disorders of the thyroid gland, as well as a number
indirect data, in particular, its abundant blood supply despite
small weight (20-30 g). The thyroid gland consists of many
special cavities - follicles filled with a viscous secretion - a colloid.
The colloid contains a special iodine-containing glycoprotein with high
pier mass - about 650,000 (5000 amino acid residues). This glyco-
the protein is called iodt andreo globulin. He is
a spare form of thyroxine and triiodothyronine - the main hormones of follicles -
the curvature of the thyroid gland.
In addition to these hormones (biosynthesis and functions of which will be considered
below), in special cells - the so-called parafollicular cells,
or C-cells of the thyroid gland, the peptide hormone is synthesized
childbirth, which ensures a constant concentration of calcium in the blood. He
received the name "calcitonin". For the first time in existence, calcite
nina, which has the ability to maintain a constant level of cal-
tion in the blood, pointed out in 1962 by D. Kopp, who mistakenly believed that this
the hormone is synthesized by the parathyroid glands. Currently
calcitonin is not only isolated in its pure form from thyroid tissue
animals and humans, but the 32-membered amino acid
consistency confirmed by chemical synthesis. Below is a
on the primary structure of thyroid-derived calcitonin