Water: electrical conductivity and thermal conductivity. Units for measuring the electrical conductivity of water. At the time of the Roman physician Dioscorides pedania, no one had a clue about the composition of soda. The electrical conductivity of baking soda
Who knows the formula of water since school days? Of course that's all. It is likely that from the entire course of chemistry, many who then do not study it in a specialized way, only knowledge of what the formula H 2 O means remains. But now we will try to understand in as much detail and deeply as possible what are its main properties and why it is life without it on planet Earth is impossible.
Water as a substance
The water molecule, as we know, consists of one oxygen atom and two hydrogen atoms. Its formula is written as follows: H 2 O. This substance can have three states: solid - in the form of ice, gaseous - in the form of vapor, and liquid - as a substance without color, taste or smell. By the way, this is the only substance on the planet that can exist in all three states simultaneously under natural conditions. For example: at the poles of the Earth - ice, in the oceans - water, and the evaporation under the sun's rays is steam. In this sense, water is abnormal.
Water is also the most abundant substance on our planet. It covers the surface of the planet Earth by almost seventy percent - these are oceans, and numerous rivers with lakes, and glaciers. Most of the water on the planet is salty. It is undrinkable and not suitable for agriculture. Fresh water makes up only two and a half percent of the total amount of water on the planet.
Water is a very strong and high quality solvent. Due to this, chemical reactions in water take place at a tremendous speed. This property also affects the metabolism in the human body. that the body of an adult is seventy percent water. For a child, this percentage is even higher. By old age, this figure drops from seventy to sixty percent. By the way, this feature of water clearly demonstrates that it is it that is the basis of human life. The more water there is in the body, the healthier, more active and younger it is. Therefore, scientists and doctors of all countries tirelessly insist that you need to drink a lot. It is water in its pure form, and not substitutes in the form of tea, coffee or other drinks.
Water shapes the planet's climate, and this is no exaggeration. Warm ocean currents heat entire continents. This is due to the fact that the water absorbs a lot of solar heat, and then gives it back when it starts to cool. This is how she regulates the temperature on the planet. Many scientists say that the Earth would have cooled down and become stone long ago if it were not for the presence of so much water on the green planet.

Water properties
Water has many very interesting properties.
For example, water is the most mobile substance after air. From the school course, many, for sure, remember such a concept as the water cycle in nature. For example: a trickle evaporates when exposed to direct sunlight, turns into water vapor. Further, this vapor, by means of the wind, is carried somewhere, collects in clouds, or even falls in the mountains in the form of snow, hail or rain. Further, a trickle again runs down from the mountains, partially evaporating. And so - in a circle - the cycle repeats itself millions of times.
Also, water has a very high heat capacity. It is because of this that water bodies, especially oceans, cool down very slowly during the transition from a warm season or time of day to a cold one. Conversely, as the air temperature rises, the water heats up very slowly. Due to this, as mentioned above, water stabilizes the air temperature throughout our planet.
After mercury, water has the highest surface tension. It is impossible not to notice that a drop accidentally spilled on a flat surface sometimes becomes an impressive speck. This is the manifestation of the viscosity of water. Another property manifests itself in her when the temperature drops to four degrees. Once the water cools down to this point, it becomes lighter. Therefore, ice always floats on the surface of the water and solidifies with a crust, covering rivers and lakes. Thanks to this, fish do not freeze in reservoirs that freeze in winter.
Water as a conductor of electricity
First, you should find out what electrical conductivity is (including water). Electrical conductivity is the ability of a substance to pass through itself electricity... Accordingly, the electrical conductivity of water is the ability of water to conduct current. This ability directly depends on the amount of salts and other impurities in the liquid. For example, the electrical conductivity of distilled water is almost minimized due to the fact that such water is purified from various additives that are so necessary for good electrical conductivity. An excellent conductor of current is sea water, where the concentration of salts is very high. Also, electrical conductivity depends on the temperature of the water. The higher the temperature, the greater the electrical conductivity of the water. This pattern was revealed thanks to the multiple experiments of physicists.

Measuring the conductivity of water
There is such a term - conductometry. This is the name of one of the methods of electrochemical analysis based on the electrical conductivity of solutions. This method is used to determine the concentration in solutions of salts or acids, as well as to control the composition of some industrial solutions. Water has amphoteric properties. That is, depending on the conditions, it is able to exhibit both acidic and basic properties - to act both as an acid and as a base.
The instrument used for this analysis has a very similar name - conductometer. With the help of a conductometer, the electrical conductivity of electrolytes in the solution being analyzed is measured. Perhaps it is worth explaining another term - electrolyte. This is a substance that, when dissolved or melted, decomposes into ions, due to which an electric current is subsequently conducted. Ion is an electrically charged particle. Actually, a conductometer, taking as a basis certain units of electrical conductivity of water, determines its specific electrical conductivity. That is, it determines the electrical conductivity of a specific volume of water, taken as the initial unit.
Even before the beginning of the seventies of the last century, the unit of measure "mo" was used to denote the conductivity of electricity, it was a derivative of another quantity - Ohm, which is the basic unit of resistance. Electrical conductivity is inversely proportional to resistance. Now it is measured in Siemens. This value got its name in honor of a physicist from Germany - Werner von Siemens.
Siemens
Siemens (it can be denoted as Cm and S) is the reciprocal of Ohm, which is a unit for measuring electrical conductivity. One cm is equal to any conductor whose resistance is equal to 1 ohm. Siemens is expressed through the formula:
- 1 cm \u003d 1: Ohm \u003d A: B \u003d kg −1 · m −2 · s³A², where
A - ampere,
V - volts.

Thermal conductivity of water
Now let's talk about whether this is the ability of a substance to transfer thermal energy. The essence of the phenomenon lies in the fact that the kinetic energy of atoms and molecules, which determine the temperature of a given body or substance, is transferred to another body or substance during their interaction. In other words, thermal conductivity is heat exchange between bodies, substances, and also between body and substance.
The thermal conductivity of water is also very high. People use this property of water every day without noticing it. For example, pouring cold water into a container and cooling drinks or food in it. Cold water takes heat away from a bottle or container, giving back cold in return; a reverse reaction is also possible.
Now the same phenomenon can be easily visualized on a planetary scale. The ocean heats up during the summer, and then - with the onset of cold weather, it slowly cools down and gives up its heat to the air, thereby heating the continents. Having cooled down over the winter, the ocean begins to very slowly warm up in comparison with the earth and gives its coolness to the continents languishing from the summer sun.

Density of water
It was said above that fish live in a reservoir in winter due to the fact that the water solidifies with a crust over their entire surface. We know that water begins to turn into ice at a temperature of zero degrees. Due to the fact that the density of water is greater than the density, it floats and solidifies over the surface.
water properties
Also, water under different conditions is capable of being both an oxidizing agent and a reducing agent. That is, water, donating its electrons, is positively charged and oxidized. Or it acquires electrons and is charged negatively, which means that it is restored. In the first case, the water is oxidized and called dead. It has very powerful bactericidal properties, but you don't need to drink it. In the second case, the water is alive. It invigorates, stimulates the body to recover, brings energy to the cells. The difference between these two properties of water is expressed in the term "redox potential".

What water can react with
Water is capable of reacting with almost all substances that exist on Earth. The only thing is that for these reactions to occur, you need to provide a suitable temperature and microclimate.
For example, at room temperature, water reacts well with metals such as sodium, potassium, barium - they are called active. With halogens it is fluorine, chlorine. When heated, water reacts well with iron, magnesium, coal, methane.
With the help of various catalysts, water reacts with amides, carboxylic acid esters. A catalyst is a substance that seems to push the components to a mutual reaction, accelerating it.
Is there water anywhere else besides Earth?
So far, no planet in the solar system, except for the Earth, has not been found. Yes, they assume about its presence on the satellites of such giant planets as Jupiter, Saturn, Neptune and Uranus, but so far scientists have no exact data. There is one more hypothesis, not yet fully tested, about groundwater on the planet Mars and on the Earth's satellite - the Moon. Regarding Mars, in general, a number of theories have been put forward that there was once an ocean on this planet, and its possible model was even projected by scientists.

Outside the solar system, there are many large and small planets where, according to scientists, there may be water. But so far there is not the slightest opportunity to be sure of this for sure.
How the thermal and electrical conductivity of water is used for practical purposes
Due to the fact that water has a high value of heat capacity, it is used in heating mains as a heat carrier. It provides heat transfer from producer to consumer. Many nuclear power plants also use water as an excellent coolant.
In medicine, ice is used for cooling and steam for disinfection. Ice is also used in the catering system.
In many nuclear reactors, water is used as a moderator for a successful nuclear chain reaction.
Pressurized water is used for splitting, breaking and even cutting rocks. It is actively used in the construction of tunnels, underground rooms, warehouses, subways.
Conclusion
From the article it follows that water, by its properties and functions, is the most irreplaceable and amazing substance on Earth. Does the life of a person or any other living creature on Earth depend on water? Of course, yes. Does this substance contribute to human scientific activity? Yes. Does the water have electrical conductivity, thermal conductivity, and other useful properties? The answer is also yes. Another thing is that there is less and less water on Earth, and even more pure water. And our task is to preserve and secure it (and therefore all of us) from disappearance.
Rozanov Evgeniy
Soda is a multifaceted substance, its use is different. Soda is used from the food industry to metallurgy. I became interested in these substances that everyone has in the house and decided to study how the various properties of an aqueous solution of soda manifest themselves depending on the temperature and concentration of the solution.
Download:
Preview:
To use the preview of presentations, create yourself a Google account (account) and log into it: https://accounts.google.com
Slide captions:
Work performed by: Rozanov Evgeniy. Academic Supervisor: Khabarova Olga Nikolaevna
Doroninskoye soda lake is a hydrological natural monument, the largest soda lake in Eastern Siberia. The area of \u200b\u200bthe reservoir in different seasons and years varies from 3.7 to 4.8 km2. The average depth of the water is about 4 m, the greatest is 6.5 m. On the lake there is the most famous deposit of self-precipitated soda in Transbaikalia.
Dioscorides Pedanius is Greek by origin, doctor, pharmacologist and naturalist, one of the founders of botany, Dioscorides Pedanius was born in Anazarba, Cilicia, Asia Minor (modern Nazarva). Dioscorides traveled a lot with the Roman army under the emperor Nero, practicing military medicine, collecting and identifying plants. The main work of Dioscorides - "De materia medica" ("On medicinal substances") contains a description of 600 plants, 1000 different medicines. In the Middle Ages, "De materia medica" was considered the main source of knowledge in botany and pharmacology.
Henri Louis Duhamel du Monceau Peter the Great
LeBlanc He studied medicine, attended lectures on chemistry by G. Ruel in the Botanical Garden of Paris. In 1791, Nicola LeBlanc received a patent for "A Method for Converting Glauber's Salt into Soda". LeBlanc offered his technology for producing soda to the Duke Philip of Orleans, whose personal physician he was. In 1789, the duke signed an agreement with LeBlanc and allocated him two hundred thousand silver livres for the construction of the plant. A soda plant in the suburbs of Paris Saint-Genis was called "Franciade - Leblanc Soda" and daily produced 100-120 kg of soda. During the French Revolution in 1793, the Duke of Orleans was executed, his property confiscated, and the soda factory and Leblanc's patent itself were nationalized. Only seven years later, LeBlanc was returned to the ruined plant, which he could not restore.
Purpose: To investigate the dependence of the electrical conductivity of an aqueous solution of baking soda on the temperature and concentration of the aqueous solution.
Objectives: To study the literature on the research topic. Conduct a knowledge survey on the various uses of baking soda. Learn to prepare a solution of baking soda of various concentrations. Explore the dependence of electrical conductivity on the concentration of the solution and temperature.
Relevance of research Soda is a multifaceted substance, its application is different. Soda is used from the food industry to metallurgy. Knowing its properties is always relevant.
Soda is a multifaceted substance
Scope of application of baking soda chemical light industry textile industry food industry medical industry metallurgy
Chemical industry In the chemical industry - for the production of dyes, foams and other organic products, fluoride reagents, household chemicals.
Metallurgy In metallurgy - in the precipitation of rare earth metals and ore flotation.
Textile and light textile industry (finishing of silk and cotton fabrics). light industry - in the production of sole rubber and artificial leather, tanning (tanning and neutralization of leather).
Food industry In the food industry - bakery, confectionery, beverage preparation.
Medical industry In the medical industry - for the preparation of injection solutions, anti-tuberculosis drugs and antibiotics
Questionnaire In what areas of industry do you think baking soda is used: Food industry Medicine Metallurgy Chemical industry Light industry At home
Poll results
Conclusion on the survey Most of the respondents answered that soda is used most often in everyday life, in the food industry, in the chemical industry.
Hypothesis If you increase the concentration of an aqueous solution of baking soda, then its electrical conductivity will increase.
Experience No. 1 "Preparation of an aqueous solution of baking soda" Purpose: to learn how to prepare an aqueous solution of baking soda of various concentrations. Equipment: 3 beakers, baking soda, filtered water, scales, weights.
No. Mass of soda (g) Mass of water (ml) Concentration of soda (%) 1 4 96 4 2 8 92 8 3 12 88 12
Conclusion: Experimentally, I learned to prepare an aqueous solution of baking soda of various concentrations.
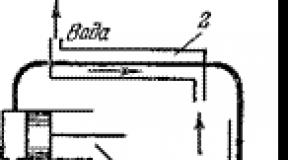
Experiment No. 2 "Investigation of the electrical conductivity of a baking soda solution" Purpose: to prove that with an increase in the concentration of a baking soda solution, its electrical conductivity increases. Equipment: Power source, 2 electrodes, 3 glasses with a solution of soda of various concentrations, ammeter, voltmeter, connecting wires, key
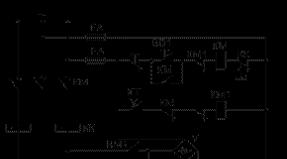
Installation diagram
Table No. Soda concentration I (A) U (B) R (Ohm) λ \u003d 1 / R (1 / Ohm \u003d Cm) 1 4 1.0 6 6 0.17 2 8 1.4 6 4.9 0.23 3 12 1.7 6 3.53 0.28
Formulas for calculating R \u003d U / I (Ohm \u003d V / A) λ \u003d 1 / R (1 / Ohm \u003d Cm) (Siemens)
Conclusion: Experimentally, I learned to determine the electrical conductivity of baking soda and made sure that the higher the concentration of the solution, the greater the electrical conductivity of the baking soda solution. And the resistance of the solution decreases with increasing concentration.
Experiment No. 3 "Investigation of the dependence of electrical conductivity on the temperature of the solution" Purpose: Make sure that the electrical conductivity of the solution depends on temperature. Equipment: Thermometer, Power supply, 2 electrodes, 3 glasses with a solution of soda of different concentrations, ammeter, voltmeter, connecting wires, key, heating element.
Table of% solution t о С solution I (A) U (B) R (Ohm) λ (Cm) 4 18 1 6 6 0.17 19 1.03 6 5.83 0.172 20 1.05 6 5.71 0.175 21 1.08 6 5.56 0.180 22 1.1 6 5.45 0.183
Graph 1. Dependence of solution resistance on temperature
Graph 2. Dependence of electrical conductivity on temperature
Conclusion: It is obvious from experience that electrical conductivity increases with increasing temperature. When heated, the speed of ions increases, thereby accelerating the transfer of charges from one point to another, from one electrode to another.
Conclusion: After studying the literature on the research topic, conducting a sociological survey, we came to the conclusion: Soda is a multifaceted substance with various properties. The resistance of a soda solution depends on its concentration. The conductivity of a solution is also concentration dependent. Electrical conductivity increases with temperature.
Thanks for your attention!
Preview:
Research work
"Study of the electrical conductivity of an aqueous solution of baking soda"
Introduction
Soda was known to man about one and a half to two thousand years BC, and maybe even earlier. It was mined from soda lakes and extracted from a few deposits in the form of minerals. The first information about obtaining soda by evaporating water from soda lakes dates back to 64 AD. Alchemists of all countries until the 18th century seemed to be a kind of substance that hissed with the release of some kind of gas under the action of acids known by that time - acetic and sulfuric. At the time of the Roman physician Dioscorides Pedania, no one had a clue about the composition of soda. In 1736, the French chemist, physician and botanist Henri Louis Duhamel de Monceau was the first to obtain very pure soda from the water of soda lakes. He managed to establish that soda contains the chemical element "Natr". In Russia, even at the time of Peter the Great, soda was called "zoda" or "itch" and until 1860 it was imported from abroad. In 1864, the first soda plant based on the technology of the Frenchman Leblanc appeared in Russia. It was thanks to the appearance of their factories that soda became more accessible and began its victorious path as a chemical, culinary and even medicine.
In industry, trade and in everyday life, several products are found under the name soda: soda ash - anhydrous sodium carbonate Na2 CO 3 , bicarbonate soda - sodium bicarbonate NaHCO3
, often called baking soda, crystalline soda Na2 CO 3 10H 2 O and Na 2 CO 3 H 2 O and caustic soda, or caustic soda, NaOH.
Modern baking soda is a typical industrial product
Currently, the world produces several million tons of soda per year for various uses.
Soda is a multifaceted substance, its use is different. Soda is used from the food industry to metallurgy. I became interested in these substances that everyone has in the house and decided to study how the various properties of an aqueous solution of soda manifest themselves depending on the temperature and concentration of the solution.
So, we had a goal:
To investigate the dependence of the electrical conductivity of an aqueous solution of baking soda on the temperature and concentration of the aqueous solution.
Tasks:
- Examine the literature on the research topic.
- Conduct a knowledge survey on the various uses of baking soda.
- Learn to prepare a solution of baking soda of various concentrations.
- Explore the dependence of electrical conductivity on the concentration of the solution and temperature.
The relevance of research:
Soda is a multifaceted substance, its use is different. Soda is used from the food industry to metallurgy. Knowing its properties is always relevant.
The slide shows the main uses of baking soda.
- chemical industry
- light industry
- textile industry
- food industry
- medical industry
- metallurgy
So, in the chemical industry - for the production of dyes, foams and other organic products, fluoride reagents, household chemicals.
- In metallurgy - during the precipitation of rare earth metals and ore flotation.
- In the textile industry (finishing silk and cotton fabrics).
- In light industry - in the production of sole rubber and artificial leather, tanning (tanning and neutralization of leather).
- In the food industry - bakery, confectionery, beverage preparation.
- In the medical industry - for the preparation of injection solutions, anti-tuberculosis drugs and antibiotics
After studying the theoretical material, I decided to ask my classmates if they know what areas of the industrybaking soda is used:
- At home
- Food industry
- Medicine
- Chemical industry
- Metallurgy
- Light industry
Here are the results of the survey: the largest number of respondents answered:
- At home -63%
- Food industry-71%
- Chemical industry - 57%, the smallest number of respondents indicated the use of soda in metallurgy and light industry.
For further research, I needed to prepare an aqueous solution of various concentrations.
Hypothesis
So, if you increase the concentration of an aqueous solution of baking soda, then its electrical conductivity will increase.
II. experimental part
"The study of the electrical conductivity of an aqueous solution of baking soda"
Purpose: make sure that in an aqueous solution of soda there are carriers of electricity - ions that conduct electric current.
Equipment: baking soda, chemical glasses made of heat-resistant glass, electrodes, connecting wires, power supply, ammeter, voltmeter, key, laboratory balance, weights, thermometer, electric stove.
Experience 1. "Preparation of an aqueous solution of baking soda"
Purpose: Learn how to prepare an aqueous solution of baking soda of various concentrations.
Equipment: chemical glasses from heat-resistant glass, filtered water, scales, weights, baking soda.
Performance experience:
- On the scales weigh 4 g of baking soda;
- Pour 96 ml into a beaker. filtered water;
- Pour soda into a glass of water and mix thoroughly;
- Repeat the experiment for the preparation of a solution of 8% and 12%
Mass of soda (g) | Amount of water (ml) | soda concentration in (%) |
|
Output: Experimentally learned to prepare an aqueous solution of baking soda of various concentrations.
Experience 2. "Study of the conductivity of a solution of baking soda"
Purpose: prove that with increasing concentration of a soda solution, its conductivity increases.
Equipment: three glasses with a solution of baking soda of various concentrations, a power source, ammeter, voltmeter, connecting wires, key, electrodes.
Specific resistance is a scalar quantity numerically equal to the resistance of a homogeneous cylindrical conductor of unit length and unit area. The greater the resistivity of the material of the conductor, the greater its electrical resistance.
The unit of resistivity is an ohm meter (1 Ohm · m).
Performance experience:
- To collect electric circuit according to the scheme;
- Place the electrodes in a beaker with a baking soda solution concentration of 4%, 8% and 12%;
- Measure the readings of the ammeter and voltmeter;
- Calculate the resistance of the solution;
- Calculate the conductivity of the solution.
Table 2.
Soda | I (A) | U (B) | R (ohm) | λ \u003d 1 R (1Ω \u003d cm) |
|
0,17 |
|||||
0,23 |
|||||
3,53 | 0,28 |
For the experiment, an electric circuit was assembled. By changing the concentration of the aqueous solution, we record the readings of the ammeter and voltmeter.
The measurements were carried out at a temperature of 180 C and atmospheric pressure 757 mm Hg
Output: Experimentally learned to determine the conductivity of baking soda and made sure that the higher the concentration of the solution, the greater the conductivity of the solution of baking soda. And the resistance of the solution, with increasing concentration, decreases. Therefore, with a 12% baking soda solution, the electrical conductivity will be the highest and the resistance the lowest.
Experience 3. "The study of the dependence of electrical conductivity on the temperature of the solution"
Purpose: Verify that conductivity changes with temperature.
Equipment: three glasses with a solution of baking soda of various concentrations, power supply, ammeter, voltmeter, connecting wires, key, electrodes, thermometer, electric stove.
Performance experience:
- Assemble the installation according to the scheme;
- Put 4% baking soda solution on the tile;
- Turn on the tile;
- Fix the temperature of the solution;
- Measure the readings of the ammeter and voltmeter through each degree of the solution;
- Calculate the resistance and conductivity using the formulas.
1,05
5,71
0,175
1,08
5,56
0,180
5,45
0,183
λ \u003d 1R (1ohm \u003d cm)
Output: From experience it is obvious that electrical conductivity increases with increasing temperature. When heated, the ion velocity increases, thereby accelerating the process of charge transfer from one point to another.
Chart 1. The dependence of the resistance of the solution on temperature.
Chart 2. Conductivity versus temperature
Conclusion
Having studied the literature on the properties of baking soda, its use in medicine, the food industry, everyday life, having done a number of experiments, we were convinced that:
- Soda is a multifaceted substance with various properties.
- The resistance of a soda solution depends on its concentration.
- The conductivity of the solution also depends on the concentration.
- Conductivity increases with temperature.
Literature
- General chemical technology. Ed. I.P. Mukhlenova. Textbook for chemical and technological specialties of universities. - M .: Higher school.
- Fundamentals of General Chemistry, vol. 3, B.V. Nekrasov. - M.: Chemistry, 1970.
- General chemical technology. Furmer I.E., Zaitsev V.N. - M.: Higher School, 1978.
- General Chemical Technology, ed. I. Wolfkovich, v. 1,Soda M. - L., 1953, p. 512-54;
- Benkovsky V., Technology of soda products, M, 1972;
- Shokin I.N., KrasheninnikovSoda A., Soda technology, M., 1975.
Research work "Study of the electrical conductivity of an aqueous solution of drinking soda"
Introduction
Soda was known to man about a half to two thousand years BC, and maybe even earlier. It was extracted from soda lakes and extracted from a few deposits in the form of minerals. The first information about obtaining soda by evaporating the water of soda lakes dates back to 64 AD. Up to the 18th century, it seemed to the alchemists of all countries a certain substance that hissed with the release of some gas under the action of acids known at that time - acetic and sulfuric. At the time of the Roman physician Dioscorides Pedania, no one had a clue about the composition of the soda. In 1736, the French chemist, doctor and botanist Henri Louis Duhamel de Monceau was first able to get very pure soda from the water of soda lakes. He managed to establish that soda contains the chemical element "Sodium". In Russia, even during the time of Peter the Great, soda was called “zoda” or “itch” and until 1860 it was imported from abroad. In 1864, the first soda factory using the technology of the French LeBlanc appeared in Russia. It was thanks to the appearance of its factories that soda became more accessible and began its victorious path as a chemical, culinary and even medicine.
In industry, commerce and in everyday life, under the name soda, there are several products: soda ash - anhydrous sodium carbonate Na2CO3, bicarbonate sodium NaCO3, often also called drinking soda, crystalline Na2CO3 10H2O and Na2CO3 H2O, and caustic soda, or NaOH. Modern baking soda is a typical industrial product.
Currently, the world produces several million tons of soda per year for various uses.
Soda is a multifaceted substance, its use is different. Soda is used from the food industry to metallurgy. I became interested in these substances, which everyone has in the house and decided to study how various properties of an aqueous soda solution are manifested depending on the temperature and concentration of the solution.
So, before us was the goal:
To investigate the dependence of the electrical conductivity of an aqueous solution of drinking soda on the temperature and concentration of an aqueous solution.
Tasks:
Study the literature on the research topic.
Conduct a survey on the knowledge of the application of various applications of baking soda.
Learn how to prepare a solution of drinking soda of various concentrations.
Investigate the dependence of electrical conductivity on solution concentration and temperature.
The relevance of research:
Soda is a multifaceted substance, its use is different. Soda is used from the food industry to metallurgy. Knowing its properties is always relevant.
The slide shows the main applications of baking soda.
chemical industry
light industry
textile industry
food industry
medical industry
metallurgy
So, in the chemical industry - for the production of dyes, foams and other organic products, fluoride reagents, household chemical goods.
In metallurgy - during the deposition of rare earth metals and ore flotation.
In the textile industry (decoration of silk and cotton fabrics).
In light industry - in the production of plantar rubbers and artificial leathers, in the leather industry (tanning and leather neutralization).
In the food industry - bakery, confectionery production, and beverage preparation.
In the medical industry - for the preparation of injection solutions, anti-TB drugs and antibiotics
After studying the theoretical material, I decided to ask my classmates if they know in which areas of industry baking soda is used:
At home
Food industry
Medicine
Chemical industry
Metallurgy
Light industry
Here are the results of the survey: the largest number of respondents answered:
In the home -63%
Food industry-71%
Chemical industry - 57%, the smallest number of respondents indicated the use of soda in metallurgy and light industry.
For further research, I needed to prepare an aqueous solution of different concentrations.
Hypothesis
So, if you increase the concentration of an aqueous solution of baking soda, then its electrical conductivity will increase.
II. experimental part
"The study of the electrical conductivity of an aqueous solution of baking soda"
Purpose: to verify that in an aqueous solution of soda there are carriers of electricity - ions that conduct electric current.
Equipment: baking soda, chemical glasses made of heat-resistant glass, electrodes, connecting wires, power supply, ammeter, voltmeter, key, laboratory balance, weights, thermometer, electric stove. Experience 1. "Preparation of an aqueous solution of baking soda"
Purpose: To learn how to prepare an aqueous solution of baking soda of various concentrations.
Equipment: chemical glasses from heat-resistant glass, filtered water, scales, weights, baking soda.
Performance experience:
On the scales weigh 4 g of baking soda;
Pour 96 ml into a beaker. filtered water;
Pour soda into a glass of water and mix thoroughly;
Repeat the experiment for the preparation of a solution of 8% and 12%
No. Mass of soda (g) Amount of water (ml) soda concentration in (%)
1 4 96 4
2 8 92 8
3 12 88 12
Conclusion: Experimentally learned to prepare an aqueous solution of baking soda of various concentrations.
Experience 2. "Study of the conductivity of a solution of baking soda"
Purpose: to prove that with increasing concentration of a soda solution, its conductivity increases.
Equipment: three glasses with a solution of baking soda of various concentrations, a power source, ammeter, voltmeter, connecting wires, key, electrodes.
Resistivity is a scalar quantity numerically equal to the resistance of a homogeneous cylindrical conductor of unit length and unit area. The greater the resistivity of the material of the conductor, the greater its electrical resistance.
The unit of resistivity is an ohm meter (1 Ohm · m).
Performance experience:
Assemble the electrical circuit according to the scheme;
Place the electrodes in a beaker with a baking soda solution concentration of 4%, 8% and 12%;
Measure the readings of the ammeter and voltmeter;
Calculate the resistance of the solution;
Calculate the conductivity of the solution.
Table 2.
No. Concentration of soda I (A) U (B) R (Ohm) λ \u003d 1 R (1 Ohm \u003d Cm) 1 4 1.0 6 6 0.17
2 8 1,4 6 4,9 0,23
3 12 1,7 6 3,53 0,28
For the experiment, an electric circuit was assembled. By changing the concentration of the aqueous solution, we record the readings of the ammeter and voltmeter.
The measurements were carried out at a temperature of 180 ° C and an atmospheric pressure of 757 mm Hg.
Conclusion: Experimentally learned to determine the conductivity of baking soda and made sure that the higher the concentration of the solution, the greater the conductivity of the solution of baking soda. And the resistance of the solution, with increasing concentration, decreases. Therefore, with a 12% baking soda solution, the electrical conductivity will be the highest and the resistance the lowest.
Experience 3. "The study of the dependence of electrical conductivity on the temperature of the solution"
Purpose: Ensure that conductivity changes with temperature.
Equipment: three glasses with a solution of baking soda of various concentrations, a power source, an ammeter, a voltmeter, connecting wires, a key, electrodes, a thermometer, an electric stove.
Assemble the installation according to the scheme;
Put 4% baking soda solution on the tile;
Turn on the tile;
Fix the temperature of the solution;
Measure the readings of the ammeter and voltmeter through each degree of the solution;
Calculate the resistance and conductivity using the formulas.
To study this dependence, a 4% percent baking soda solution was heated by fixing the temperature with a thermometer.
Table 3.
% solution to С solution I (A) U (B) R (Ohm) λ (cm)
4 18 1 6 6 0,17
19 1,03 6 5,83 0,172
20 1,05 6 5,71 0,175
21 1,08 6 5,56 0,180
22 1,1 6 5,45 0,183
λ \u003d 1R (1ohm \u003d cm)
Conclusion: It is obvious from experience that electrical conductivity increases with increasing temperature. When heated, the ion velocity increases, thereby accelerating the process of charge transfer from one point to another.
Chart 1. The dependence of the resistance of the solution on temperature.
Chart 2. Conductivity versus temperature
Conclusion
Having studied the literature on the properties of baking soda, its use in medicine, the food industry, everyday life, having done a number of experiments, we were convinced that:
Soda is a multifaceted substance with various properties.
The resistance of a soda solution depends on its concentration.
The conductivity of the solution also depends on the concentration.
Conductivity increases with temperature.
Literature
General chemical technology. Ed. I.P. Mukhlenova. Textbook for chemical and technological specialties of universities. - M .: Higher school.
Fundamentals of General Chemistry, vol. 3, B.V. Nekrasov. - M.: Chemistry, 1970.
General chemical technology. Furmer I.E., Zaitsev V.N. - M.: Higher School, 1978.
General Chemical Technology, ed. I. Wolfkovich, v. 1, Soda M. - L., 1953, p. 512-54;
Benkovsky V., Technology of soda products, M, 1972;
Shokin I.N., Krasheninnikov Soda A., Soda technology, M., 1975.
Water is a unique substance that has a complex molecular structure, not yet fully understood. Regardless of the state of aggregation, H2O molecules are firmly connected to each other, which determines the set physical properties water and its solutions. Let's find out if ordinary water has thermal and electrical conductivity.
The main physical properties of H2O include:
- density;
- transparency;
- colour;
- smell;
- taste;
- temperature;
- compressibility;
- radioactivity;
- heat and electrical conductivity.
Recent characteristics of the thermal conductivity and electrical conductivity of water are very unstable and depend on many factors. Let's consider them in more detail.
Electrical conductivity
Electric current is a one-way movement of negatively charged particles - electrons. Some substances can carry these particles, and some cannot. This ability is expressed in numerical form and represents the value of electrical conductivity.
There is still debate about whether pure water has electrical conductivity. It can conduct current, but very poorly. The electrical conductivity of the distillate is explained by the fact that H2 O molecules partially decompose into H + and OH– ions. Electroparticles move with the help of positively charged hydrogen ions, which are able to move in the water column.
What determines the electrical conductivity of a liquid
The electrical conductivity of H2 O depends on factors such as:
- the presence and concentration of ionic impurities (mineralization);
- nature of ions;
- fluid temperature;
- viscosity of water.
The first two factors are decisive. Therefore, by calculating the value of the electrical conductivity of the liquid, we can judge the degree of its mineralization.
There is no pure water in nature. Even the spring is a kind of solution of salts, metals and other electrolyte impurities. First of all, these are Na +, K +, Ca2 +, Cl-, SO4 2-, HCO3 - ions. Also, its composition may include weak electrolytes, which are unable to strongly change the ability to conduct current. These include Fe3 +, Fe2 +, Mn2 +, Al3 +, NO3 -, HPO4 - and others. They can exert a strong influence on electrical conductivity only in the case of a high concentration, as, for example, sewage with production waste. Interestingly, the presence of impurities in water, which is in a state of ice, does not affect its ability to conduct electricity.
Electrical conductivity of sea water
Sea water is better able to conduct electric current than fresh. This is due to the presence in it of a dissolved NaCl salt, which is a good electrolyte. The mechanism of increasing conductivity can be described as follows:
- When dissolved in water, sodium chloride decomposes into Na + and Cl- ions, which have different charges.
- Na + ions attract electrons because they have the opposite charge.
- The movement of sodium ions in the water column leads to the movement of electrons, which, in turn, leads to the appearance of an electric current.
Thus, the electrical conductivity of water is determined by the presence of salts and other impurities in it. The smaller they are, the lower the ability to conduct electric current. In distilled water, it is practically zero.
Conductivity measurement
The conductivity of solutions is measured using conductometers. These are special devices, the principle of which is based on the analysis of the ratio of electrical conductivity and concentration of electrolyte impurities. Today, there are many models that can measure the electrical conductivity of not only highly concentrated solutions, but also pure distilled water.
Thermal conductivity
Thermal conductivity is the ability of a physical substance to conduct heat from heated parts to colder ones. Water, like other substances, has this property. Heat transfer occurs either from molecule to molecule H2 O, which is a molecular type of thermal conductivity, or when moving fluid flows - a turbulent type.
The thermal conductivity of water is several times higher than that of other liquid substances, with the exception of molten metals - they have this indicator even higher.
The ability of water to conduct heat depends on two factors: pressure and temperature. With increasing pressure, the conductivity increases, with increasing temperature to 150 ° C it increases, then it begins to decrease.
Why does the pool water seem cold to us?
The thermal conductivity of water is several tens of times higher than that of air. When a person is immersed in water or just poured over it, heat loss increases, so it becomes much colder than in air at the same temperature. This can be seen in the examples given in the table:
The most interesting facts about water: Video
What is the difference between an electrode and a heating boiler?
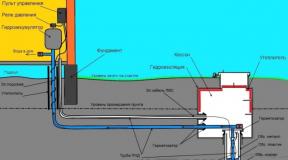
In a heating element with the help of electric energy, a heating element is heated - a tubular electric heater, which then transfers its heat to the heat carrier. The electrode boiler works by passing current through the coolant (water or non-freezing coolant "-20 C"). Walkthrough alternating current can not be called electrolysis, since only ionization of the liquid occurs. The electrode boiler is a simple and very reliable water (liquid) heater, in ideal cases it can work without replacing the elements for many years (decades).
What affects the performance and service life of electrode boilers?
For the operation of the electrode boiler, it is necessary that the coolant has the desired specific resistance (specific conductivity). The electrode boiler is part of the heating system. To ensure reliable, long-term, trouble-free operation of the boiler, the heating system must comply with the recommended parameters in the boiler passport.
Why are heating systems based on electrode boilers generally more economical and reliable than heating elements?
Despite some difficulties when starting heating systems based on electrode boilers, electrode boilers are more economical than heating elements by at least 20-30%. The efficiency of electrode boilers is tested by the practice of installation and operation for more than 15 years. Reliability and profitability is provided by a simpler, reliable design. In a heating element, the heating elements are first heated, and then the heating elements give off heat to the liquid. In the electrode boiler, the role of the heater is played by the liquid itself. With the passage of current, the liquid is heated with the entire volume in the boiler. Using electrode heating of the liquid, it is possible to reduce the volume of the boiler several times in comparison with a heating element of the same power.
With a correctly assembled system, the boiler starts with a small (less than 50%) of the rated power, and when it warms up, it gradually gains power. Modern automation allows you to maintain a comfortable room temperature with an accuracy of +/- 0.2 degrees. C. When heating country houses, it is possible to use the weekly mode to control the heating system. Thus, the efficiency in the operation of electrode boilers is achieved by:
- Less inertia of heating (several times);
- Smooth start;
- The use of modern automation;
Reliability and durability is ensured by the simplicity of design and the use of modern materials.
How much electricity will the boiler consume?
The boiler will consume exactly as much electric power. energy, how much heat loss is a building.
In normal operation, with normal heat loss, with the correct choice of the boiler, with the maximum winter mode (when on the street for Kiev -23, during normal assembly of the heating system, the boiler works for about 8 hours a day (in the on - heating, shutdown - cooling) mode Next, we take the boiler power and multiply it by an average of 8 hours and get the consumption of electric energy per day.
How to choose the right boiler?
The electrode boiler "ION" is selected according to the following parameters:
- 1 kW of electrode boiler power can heat a room with an area of \u200b\u200bup to 20 sq / m, a volume of up to 60 cubic meters / m and 40 liters of water in the heating system.
For example, a boiler with a capacity of 5 kW can heat a room with an area of \u200b\u200b100 sq / m, a volume of 300 cubic / m and with the amount of water in the heating system up to 240 liters.
What pipes and radiators can be used in a heating system with an "ION" electrode boiler?
For heating systems, any pipes that are certified for this purpose can be used. We recommend the use of polypropylene.
The use of plastic pipes is undesirable, connecting fittings significantly narrow the bore;
a metal-plastic pipe is often prone to deformation and delamination when fluid temperature fluctuates.
You can use any modern radiators (cast iron, bimetallic), but it is best to use steel batteries. Cast iron radiators are undesirable, since they have a significant amount of fluid, a porous structure and contain dirt inside.
To ensure the durability and reliability of the boiler, the inner diameter of the inlet and outlet pipes and pipe fittings should not be less than the inner diameter of the inlet and outlet pipe of the boiler itself.
What are the advantages of electrode boilers "ION"?
The working chamber of ION boilers is made of thick pipe special material, which is very important for ionization boilers in terms of their reliability and durability.
The working chamber of almost all such boilers is made of thin-walled ordinary pipe material. The electrodes of larger diameter ION boilers are made of a special alloy, which increases their durability and reliability during the ion-exchange process, and also makes it possible to form a heat flow inside the boiler chamber at a higher speed, unlike boilers of the same boilers of other manufacturers.
ION boilers are represented in a wider model range, unlike other brands of boilers, which allows to expand customer demand.
The boiler manufacturer “ION” does not tie the customer to its heat carrier, and the “ION” electric boilers can be operated, unlike some boilers, with ordinary water or with independently prepared solution in the heating system.
Can I use antifreeze as a coolant?
It must be understood that antifreeze is not intended for use in heating systems. He is poisonous! It is better to use special non-freezing liquids. But since the manufacturers of these fluids do not take into account its electrical conductivity, it is possible, after pumping it into the heating system, you still have to do the preparation - set the electric boiler to the required current (this is described in detail in the instruction manual). From practice, I can say that usually when using non-freezing liquids, the current in the phase of the electric boiler is overestimated, and it is necessary to dilute with distilled water (approximately to a freezing temperature of -5-10 g.).
And of course, do not forget about the properties of antifreeze:
- The physical properties of antifreeze are significantly different from the physical properties of water. The heat capacity of antifreezes is 15-20% less than that of water, the viscosity is 2-3 times higher, the volume expansion is 40-60% more. The values \u200b\u200bof thermal conductivity, boiling point, and other physical characteristics also differ. This means that when using antifreeze in the heating system, it will be necessary to increase the heat output of radiators by 40–50%, increase the volume of the expansion tank by 40–50%, increase the pressure of the circulation pump by 60%, and change a number of other parameters of the heating system, including and boiler power.
- If the temperature of antifreeze in the system, even at any one of its points (and most often it occurs inside the boiler’s heating element), exceeds the critical value for this brand of antifreeze - thermal decomposition of ethylene glycol and anticorrosion additives occurs with the formation of acids and precipitation of solid precipitation. Precipitation degrades the flow of coolant through the system. Acids corrode metals in the heating system. Also, overheating of antifreeze causes increased foaming, which leads to airing of the system, and in some cases to thickening of the foam, and the formation of hard foam-like deposits. Decomposition of additives leads to the fact that antifreeze enters into a chemical reaction with the materials of sealants - rubber, paronite, etc., which caused a leak at the joints. In addition, the use of pipelines having an internal zinc coating is unacceptable.
- Antifreezes have the property of increased permeability or fluidity. More than threaded connectionsgaskets, seals, the higher the likelihood of leakage. Basically, a leak often occurs when the heating is off, when the system has cooled down. Due to cooling, there is a decrease in the volume of metal compounds and, as a result, the appearance of microchannels through which antifreeze flows out. For this reason, all connections in the heating system must be accessible for inspection and repair, which means that hidden installation of the heating system is unacceptable. Ethylene glycol-based antifreezes are toxic (a one-time lethal dose of 100-300 ml), therefore they cannot be used for heating water in hot water systems, since when the heat exchangers are leaking, they can get to the points of analysis of hot water. Antifreeze vapors are also toxic and must not enter residential premises.
- If you have no other choice and you decide to use non-freezing fluid as a coolant, you should opt for non-freezing fluid for POTOK-40 electrode boilers, but you should take into account that for this it is necessary to replace all rubber gaskets in the system heating on paronite!
Is it possible to use the electrode boiler "ION" in conjunction with a circulation pump?
The electrode boiler is a flow type heater and for the correct operation of the boiler and heating system using a circulation pump, it is necessary to provide a coolant flow through the boiler with the following indicators:

Are pipes of any diameter used when installing an electrode boiler?
In the heating system, it is recommended to do the wiring at the inlet and outlet of the electric boiler with pipes of at least 1 inch in diameter in the heating system. After the comb, you can switch to pipes of smaller diameter, provided that the total cross-section of pipes of smaller diameter is at least 1 inch.
How to heat a house with an area of \u200b\u200bmore than 750 kV / m?
What should I do if the area of \u200b\u200bmy premises is 2800 kV / m?
For an area of \u200b\u200b2800 kV / m, it is necessary to install a mini-boiler room, consisting of 4 electrode boilers "ION" 3/36 connected in parallel with each other. With the parallel inclusion of two or more electric electrode boilers "ION" (of the same power) in one water heating system, the area (volume) of the heated room increases by 2 or more times.
For example: two 3/36 modifications heat an area of \u200b\u200b1500 sq / m with a volume of 4500 cubic meters, three modifications 3/36 heat an area of \u200b\u200b2250 kV / m, a volume of 6750 cubic meters, etc.
Can an electrode boiler work without a circulation pump?
The ionization chamber, where the heating process takes place, is small in size, therefore, a sharp heating of the coolant and, as a consequence, an increase in its pressure (with a maximum power of the device up to 2 atmospheres) follow. Thus, the "ION" electrode boiler can operate in heating systems without a circulation pump, if the heating system is assembled according to the natural circulation scheme.
Is it possible to connect in parallel with other boilers?
It is possible to mount the electrode boiler in parallel with other boilers (gas, solid fuel, etc.), and use them at a convenient time for you.

Do you need an ammeter or measuring clamps to start the electrode boiler?
After connecting the boiler to the heating system and turning on the power, the ammeter measures the current consumption. If the current strength is greater than that specified in the boiler passport, it is necessary to add distilled (melt or rain) water to the heating system. If the current is less than required, it is necessary to add caustic (baking) soda to the heating system at the rate of 30 grams per 100 liters of water, stirring the soda in warm water.
Is it possible to use the "ION" electrode boiler in heating systems with aluminum radiators?
Yes, it’s possible, the only caveat is that instead of soda solution, it is necessary to use ASO-1 (a special tool for aluminum radiators) to increase the electrical conductivity of water
What liquid is used in the heating system during the operation of the electrode boiler "ION"?
When the electrode boiler "ION" is operating, a specially prepared heat carrier is not needed. In his work, ordinary water is used with a specific electrical resistance of not more than 1300 Ohm cm. Since water is an element of an electrical circuit that generates heat, it needs some preparation to obtain the necessary electrical resistance (for example, attempts to heat distilled water will not be successful, since it does not conduct electric current). Preparation is carried out empirically - they reduce the electrical resistance of water by adding a solution of caustic (baking) soda, or increase by mixing distilled (rain, melt) water. All this is described in detail in the passport for electric boilers.
Is it possible to use the "ION" electrode boiler for hot water supply?
Electrode boilers “ION” can work together with indirect heating boilers to obtain hot water supply, for example, the “ION” 3/9 electric boiler can heat a room with an area of \u200b\u200bup to 180 m2, a ceiling height of up to 3 meters and a water volume in the heating system of up to 360 liters, with When connecting an indirect heating boiler, you need to add power to it for hot water supply (DHW) based on the passport data of your boiler, for example 3/6 kW, total for heating a house and hot water, you need a boiler with a total power of 3/9 kW + 3/6 kW \u003d 3 / 15 kW
Is it possible to use the electric electrode boiler "ION" in conjunction with the "warm floor" system?
Water underfloor heating is a closed system of pipes located in the floor screed and connected to the heating system. Metal-plastic pipes are usually used because of the ease of installation. Underfloor heating can be used as primary or secondary heating. When using a warm floor with an "ION" electric electrode boiler, a greater economic effect can be achieved.
A warm water floor has a number of advantages. Due to the large surface, the amount of radiated heat increases and is immediately transferred to surrounding objects. Thus, the warm floor provides an even horizontal and vertical distribution of heat over the entire area of \u200b\u200bthe room.
Can you explain in an accessible language how to prepare a coolant?
If you use ordinary water in your heating system as a heat carrier, then it must be brought into compliance with GOST R 51232 "Drinking water" (1300 Ohm per cubic cm).
At home, you cannot do this without special equipment. But it is possible to go the other way.
When starting the “ION” electric boiler, it is necessary to measure the starting current with an ammeter using clamp meters (or a direct-on ammeter).
If at startup the current does not match the parameters specified in the product passport, then the following steps should be taken:
- The current is less - you need to add soda solution in portions (it reduces the resistivity of the liquid). The first stage - no more than a teaspoon per hundred liters of water (coolant). If after 2 hours the current has increased slightly, the first step should be repeated.
- The current is greater - add distilled or rain (melt) water (it increases the resistivity of the liquid).
Tell me what else you need to buy materials and still have to do to put your boiler into operation?
An approximate list of additional materials and equipment for installation and commissioning of a single-phase heating system "ION".
Required :
- Magnetic starter (contactor) corresponding to the current characteristics of this ION model.
- Automatically a single-pole circuit breaker (automatic circuit breaker) corresponding to the current characteristics of this ION model.
- Electrical cable (electric wire) according to the cross-section corresponding to the current characteristics of this ION model. Electric cable (electric wire) for connecting the thermostat (for example 3x0, 5 (0.75) or 3x0.5, pv (0.75).)
- ASO -1 (a substitute for soda for aluminum radiators), if aluminum radiators are installed in the system, to increase the electrical conductivity of water
- Box (box) for installation of start-up protection equipment.
- Direct-on ammeter (measuring clamps) for monitoring the workload and, if necessary, timely adjusting the conductivity of the coolant.
- The control lamp indicates the state of the boiler (heating, interruption, absence / presence of power supply in the network).
- SALUS FL091 weekly programmer for additional energy savings and more comfortable use of the heating system
Protective grounding MANDATORY!
Heating system:
To facilitate the operation of the ION boiler and significant energy savings, it is advisable to use a circulation pump. Provide additional heating system with valves for convenient maintenance, installation and dismantling of the boiler and pump.
And what is better three-phase boilers?
It all depends on what kind of voltage you have - 220 or 380.
If you have the opportunity to install the boiler in three phases 380V. , From 3/6 kW, this gives you additional benefits. Three-phase boilers have three electrodes that can be switched on in steps, for example, a 3/6 kW ION boiler has three 2 kW electrodes each, during off-season periods when + 10 degrees outside, you do not need to turn on the boiler at full power, and just turn on one electrode. If you do not have three phases, then you can install a three-phase boiler in one phase. The phase is divided into three outputs and connected via machines to three electrodes. Three-phase boilers it is advisable to use from 100 sq.m.
What problems can be when installing copper pipelines?
When assembling a heating system from copper pipelines, an important problem is the connection of copper with other metals in one water circulation system. In the case of direct connection of copper with steel, galvanized steel or aluminum, an electrochemical reaction occurs, causing the rapid dissolution of iron, zinc and aluminum. And also it is impossible to use pipes as an element of grounding of electrical engineering. To eliminate this phenomenon, it is necessary to separate these metals from copper with an insulating gasket. Even in the absence of a metal joint, copper stimulates corrosion of the above materials. This process is the result of the precipitation of copper ions (Cu2 +), penetrating into the water in the process of uniform corrosion of copper surfaces. Ions are deposited in places of already existing corrosion ulcers, cause accelerated destruction of the main material (steel, galvanized steel, or aluminum). The most dangerous forms of corrosion include ulcerative and erosive.
Peptic corrosion, there is local corrosion of the metal, occurs at the sites of destruction of the oxide protective film covering the pipe surfaces that are in contact with water. In cold and hot water pipes, the following factors make it difficult to form a protective film or damage an existing film:
- wrong chemical composition copper
- improper preparation of the inner surfaces of the pipes during their production,
- solder leakage on the inner surface of the pipes,
- the presence inside the pipes of solid particles (for example, sand) that penetrated the installation during installation or during operation (hence the requirement of filtering the water both supplied to the system and used to flush it).
Erosive corrosion causes a turbulent flow of water at the pipe walls. Thus, it is important to comply with the design speed of the water flow, as well as the exclusion of local resistances, such as narrowing, influx from solder, improperly performed bends.
In heating systems, the combination of steel and copper is permissible only when the oxygen content in the water does not exceed 0.1 mg / dm3, which is practically possible only in closed systems. Even in a closed circulation system, it is not recommended to use copper and aluminum radiators in the same circuit.
Can I use an electrode boiler for heating if I have a residual current device (RCD) installed in my mains?
The practical value of current leakage is determined by the design of the insulators and lies in the range of 20 ¬ 40 mA. Particular attention should be paid to this when connecting the heaters to the electric network with a residual current device (RCD) installed, which usually record a current leak within 30 ¬ 40 mA.
Given this, heaters of this type must be connected through a separate circuit breaker, bypassing the RCD.
Can I get a certificate of conformity for your products?
Our company has fifteen years of experience in the development and production of electrode (ion) boilers. For the first time in the Ukrainian market we present the new generation energy-saving electrode heating device "ION".
Manufactured using the latest technology and modern materials. Improved design and improved composition of the alloy electrode determine the long term of use.
The electrode heater "ION" is made according to the technical conditions and design documentation.
You can get acquainted with the quality certificate by clicking on the image.